Abstract
Invasive alien species could generate a multitude of impacts towards native species. The introduction and spread of Vespa velutina in Europe is raising concern for the conservation of insect’s biodiversity, including wasps due to predation, competition or a combination of these two mechanisms. Nevertheless, most evidence for negative effects on other wasps are based on laboratory experiments, direct observations, and on considerations about the biology and ecology of Vespidae. No field study in Europe explored how the abundance of V. velutina could affect the population of native Vespidae, as expected in case of competition and predation. We analysed how the abundance of V. velutina influenced that of Vespa crabro, 4 years after the arrival and establishment of V. velutina in our study area, in Italy. Moreover, we compared the abundances of three native Vespidae (V. crabro, Vespula vulgaris, Vespula germanica), between our study area and an adjacent uninvaded area with similar environmental conditions. Bayesian Generalized Linear Models revealed that the abundance of V. velutina and V. crabro was positively associated, where V. velutina was scarce. Covariation disappeared only at those trapping sites where V. velutina was extremely abundant. Moreover, abundances of native wasps were similar between the invaded and the uninvaded areas.
Implications for insect conservation
The wide-scale monitoring activity performed to investigate the effects of V. velutina on native wasps has not detected any negative effects in relation to the presence of the invasive species. More effort is however requested for understanding if V. velutina could really affect native Vespidae at the population-level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological invasions are a global driver of change, whose frequency and magnitude are increasing, due to the extended global circulation of people and trades (Simberloff et al. 2013; Seebens et al. 2017). Invasive alien species can affect the population dynamics of native species, in their invaded range, sometimes to the point of their complete replacement (Mckinney and Lockwood 1999; Säterberg et al. 2013), with consequences for communities and ecosystems (Kumschick et al. 2015; Cameron et al. 2016; Carbonell et al. 2017; Stoett et al. 2019). Among alien terrestrial insects, social wasps are particularly successful invaders (Beggs et al. 2011), which were found to outcompete native arthropods and produce large-scale ecological changes on many different occasions (Beggs 2001; Snyder and Evans 2006). This success depends upon the biological traits of social wasps, such as their high reproduction rates, their dispersal abilities, and their flexible habitat and dietary requirements (Moller 1996; Beggs et al. 2011).
The European invasion of the Asian yellow-legged hornet (Vespa velutina) is a good example of how social wasps can become successful invaders. Following its introduction to France, in 15 years the species spread and established viable populations across Central and Mediterranean Europe (Arca et al. 2015; Laurino et al. 2020). Such a rapid invasion was due to the capacity of V. velutina to use natural and human-mediated dispersal (Robinet et al. 2019). The invasion of V. velutina in Europe raised various concern, mostly related to beekeeping (Requier et al. 2019; Laurino et al. 2020) or the economic cost of its management (Barbet-Massin et al. 2020), and in 2016 the species was included in the first list of invasive species of Union concern (EU Regulation n. 1141/2016). However, while available evidence about the socio-economic impacts of V. velutina was sufficient to its inclusion in European policymaking, its impacts on native insects other than honey bees remained relatively unexplored.
Vespa velutina has a semi-specialised diet, centred on honey bees and other insects including social wasps, especially smaller species of the genus Vespula, such as Vespula germanica and Vespula vulgaris (Villemant et al. 2011a; Monceau et al. 2014; Islam et al. 2015; Rome at al. 2021; Verdasca et al. 2022). Due to its food spectrum, it has been hypothesized that V. velutina could impact on native European Vespidae due to competition, predation or a combination of these two mechanisms (Beggs 2001; Crowder and Snyder 2010; Monceau et al. 2014), like in other parts of its invaded range (e.g. Japan, Ikegami et al. 2020). In Mediterranean Europe, native Vespidae that could potentially be impacted by V. velutina belong to the genus Vespa, Vespula, Dolichovespula or Polistes. Furthermore, V. velutina is particularly likely to be a successful competitor for the native congener, the European hornet (Vespa crabro), due to: (i) the considerable dietary overlap for protein and sugar resources (Cini et al. 2018); (ii) smaller levels of boldness, exploration and activity scores for V. crabro queens (Monceau et al. 2015a); (iii) a later seasonal emergence of V. crabro compared to that of V. velutina, which could then early exploit food resources (Monceau et al. 2015b); (iv) partial overlap (Bessa et al. 2016; Franklin et al. 2017) and possible competition (Spradbery 1973; Edwards 1980) in nesting site preferences, although V. crabro is restricted to cavities or sheltered sites; (v) higher reproductive potential of V. velutina queens (Poidatz et al. 2018).
Even by not considering apparent competition, for example mediated by a pathogen (Strauss et al. 2012), V. velutina seems to be capable to directly compete with V. crabro and impact on Vespula spp. due to predation. Laboratory and observational studies have described V. velutina traits that offer the basis for hypothesizing a negative effect on native Vespidae (Cini et al. 2018; Rome et al. 2021). However, evidences from field-based studies are scarce and limited to conclusions based on overlap in temporal distribution or traits (Monceau et al. 2015b; Kwon and Choi 2020), or derived from the evaluation of habitat requirements and the spatial distribution of the two species (Choi et al. 2012; Bertolino et al. 2016; Monceau and Thiéry 2017; Rojas-Nossa et al. 2018; Rodríguez-Flores et al. 2019). Furthermore, a recent analysis on interspecific hierarchies revealed that V. crabro is able to outperform V. velutina (Kwon and Choi 2020) in direct fights.
In this study, we aim to test if the abundance of V. velutina influenced that of V. crabro, in an Italian valley where V. velutina was well-established at the time of the study. We explicitly hypothesized that V. velutina had a causal effect over V. crabro, due to niche overlap. Notably, we tested for the hypothesis that the abundance of V. velutina negatively influenced the abundance of V. crabro. To identify this causal effect in an observational setting, like our field study, where species were not manipulated, we accounted for spurious correlation by controlling for relevant environmental confounders. As complementary information, we also evaluated whether the abundances of multiple native Vespidae, V. crabro, V. vulgaris and V. germanica, differed between trapping sites in the invaded and the non-invaded area.
Materials and methods
Study area and data collection
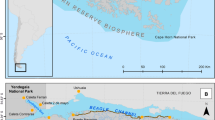
The study was carried out in the western Liguria, Italy, in an area that borders with France (Fig. 1). The climate zone is Mediterranean (Cs following Köppen Climate Classification) with dry summer and cold and wet winter and an average annual temperature of about 15 °C. Initially, two study areas were selected, corresponding to two river basins, with a distance between them of about 50 km. The two areas shared similar topographical characteristics and land cover, being covered mostly by young woodlands (Appendix S1). The two basins consisted of river valleys with a length of about 20 km, spanning from mountains to the coast, and including an elevation range between 0 and 1300 m a.s.l. At the time of the study, in 2018, one basin had not been invaded by V. velutina yet, with few records of individuals and none detected nests, while the other one had been widely colonised by V. velutina at least since 2015. Indeed, in the invaded basin, 103 V. velutina nests were detected in the year when the experiment was carried out. These areas have been selected for their wide range of elevations and land covers, for having a good road network and, most of all, for their location with respect to the diffusion of V. velutina.
For each river basin, we selected 60 sampling points based on a stratified sampling design that considered the following criteria: (i) land cover, classified upon the Corine Land Cover classification (woodlands, urban and agricultural areas); (ii) elevation, with areas divided into three classes of 250 m between 0 and 750 m a.s.l.; (iii) road network proximity, for experiment feasibility reasons. We considered 750 m a.s.l. as the upper limit for V. velutina nesting in Mediterranean areas (Villemant et al. 2011b; Bertolino et al. 2016; Rodríguez-Flores et al. 2019).
The study lasted from the end of August until the end of November 2018. Sampling points were visited approximatively every 2 weeks, in relation to weather conditions. In both valleys, sampling of Vespidae was carried out with bottle traps commonly used for monitoring social wasp species. These were transparent water bottles in PET rigged with a patented closure, activated with 0.2 l of beer as bait, and they were suspended with an iron wire at about 1.7 m off the ground (Demichelis et al. 2014). Those traps are one of the most widely used tools for hymenopterans trapping (Bacandritsos et al. 2006; Dvořák and Landolt 2006; Sorvari 2013; Lioy et al. 2020). Carbohydrate-bait traps are effective both for spring and late summer sampling, especially for trapping foundress queens in the first period and for workers or reproductive castes searching for sugar in the second period (Monceau et al. 2014, 2015b; Islam et al. 2015). The survey was carried out only in the second period to avoid any potential bias due to spring-trapping influence on nest foundation success and, therefore, on wasp abundance. Sampling lasted 81 days in the invaded valley and 88 days in the uninvaded one. At every sampling visit, we emptied the traps and renewed the bait. Collected Vespidae specimens were recorded, identified to species level by means of dichotomous keys (Buck et al. 2008) and then deposited in the collection of the Department of Agriculture, Forest and Food Sciences of the University of Turin.
Location of the study region and of the two basins (V. velutina invaded/uninvaded) where the sampling was performed. Triangles indicate the position of the sampling traps. The red–brown area is the area colonised by V. velutina before the experiment was carried out according to a range analysis of V. velutina colonies (see Bertolino et al. 2016 and Lioy et al. 2019 for insights on the methodology)
Relationship between V. crabro and V. velutina
To better highlight the relationship between V. crabro and V. velutina, which could have been masked by the absence of the latter in the uninvaded area, we first used data from traps in the invaded area only. We calculated the cumulative abundance of the two species at each trap, by considering only those traps who sampled for more than 70 days (n = 58), to avoid temporal mismatching. Then, we calculated daily abundances for the two species, by dividing trap-specific cumulative abundances per the trapping effort of each trap, in days. Daily abundances were then centred and standardized (Schielzeth 2010).
In this research, we adopted a causal inference framework, to equate the association between V. velutina and V. crabro to the causal effect of V. velutina abundance to that of V. crabro. As we already specified in the introduction, if V. velutina is much more prolific and supposed to outcompete V. crabro then we expected that the association between the two species, in a short timespan like the one of our study, will reflect a directional causal effect. Usually, the coexistence of two species in time, or the facilitating effect of the species A over the species B, is reflected into a positive association between their abundances, in cross-sectional data. On the other hand, when species A outcompetes species B, their abundances are usually negatively associated, or there is a non-linear association, with values of A which at some point stop being positively associated to those of B (Reitz and Trumble 2002; Kumschick et al. 2015).
To identify causal effects in observational settings, where data cannot be manipulated, it is important to control for potential confounders (the “back-door criterion”; Pearl 1995; Pearl and MacKenzie 2018), which could affect both the treatment (V. velutina) and the outcome variable (V. crabro). Based on the available literature, we included the following variables as potential confounders: the median Normalized Difference Vegetation Index (NDVI), the average number of nests of V. velutina around the traps between years 2016 and 2018, the median slope and aspect values of the terrain around the trap, the elevation of the trap, the Euclidean distance between the trap and the nearest water body, the average density of bee colonies in the municipality where the trap was located, the area covered by olive groves around the trap and the diversity of land cover types around the area. NDVI, the average number of nests, median slope and aspects, olive groves coverage and land cover diversity were calculated over a 500 m radius around each trap. The rationale for covariate inclusion and our causal directed acyclic graph (DAG) is provided in the supplementary material (Appendix S1).
To estimate the causal effect of the abundance of V. velutina over the abundance of V. crabro, we adopted a Bayesian Generalized Linear Model with a Gamma distribution of the error, a log-link and a moderately informative prior distribution for regression coefficients (Lemoine 2019), standardizing both predictors and the response variable. The model was fitted with four MCMC chains with 5000 iterations and a burn-in of 1000 iterations each. To explore model fitting, we checked for particular patterns in the association between standardized model residuals and fitted values. We also tested for spatial correlation in model residuals, by inspecting the Moran’s semivariogram. A complete description of model fitting and diagnostics is available in the supplementary material (Appendix S1).
Differences in the abundance of native Vespidae between the invaded and the uninvaded area
To obtain a more comprehensive picture about the impact of V. velutina over native wasps, we compared the abundance of three native species of Vespidae (V. crabro, V. germanica and V. vulgaris) between the invaded and the uninvaded area.
Based on a k-means cluster analysis of environmental covariates surrounding the traps at the two areas, we identified two different clusters of trapping sites, characterized by different environmental conditions. However, the two clusters had a very similar distribution between the two areas, indicating that, overall, environmental conditions between the two areas did not differ markedly (Appendix S1). The environmental similarity between the two areas enabled to give additional information about the effect of the long-term presence of V. velutina over the abundance of the three native species, reducing environmental conditions biases. This approach was adopted since the number of traps that did not catch any individual of V. germanica (n = 35) and V. vulgaris (n = 21) was too high for modelling their association with V. velutina in the invaded area, like in the case of V. crabro. Moreover, by considering data from the two areas, we also had a secondary source of information about the competition between V. velutina and V. crabro, which could integrate the findings about the co-occurrence of the two species measured at trapping sites.
As we did not have any baseline knowledge on the abundances of wasp populations in the two areas to hypothesize differences, nor to calculate statistical power, we did not carry out any statistical test to see whether differences were significant. However, we explored the distribution of catches between the two areas, through boxplots and calculated the overlap of their distributions, through a kernel analysis, to see how the distributions of daily catches for the three species were similar between areas.
Results
A total of 6632 Vespidae were collected in the two valleys, belonging to five species: V. crabro (n = 4721), V. velutina (n = 1452), V. germanica (n = 317), V. vulgaris (n = 141) and Dolichovespula media (n = 1). In the invaded area, V. crabro was always dominant over V. velutina (percentage among Vespidae respectively 62.6% and 33.4%) and the two hornet species were caught in all traps, except for 1 and 2 traps respectively for V. crabro and V. velutina. Few individuals of V. velutina (n = 26) were caught in the uninvaded area. Focusing on the effect of V. velutina on V. crabro, our best candidate model explained approximately 44.8% of the variability in the abundance of V. crabro. We did not detect any pattern when comparing model residuals to fitted values, and the Moran’s semivariogram did not indicate the existence of isotropic spatial correlation between the observations (Appendix S1). The model had a quadratic polynomial term linking the abundance of V. velutina to the abundance of V. crabro. Initially, the relationship between the two species was moderately positive, however, for high values of V. velutina, the two species did not covary anymore and the curve reached a plateau (Fig. 2).
K-means cluster analysis revealed that the environmental characteristics of trapping points between the invaded and the uninvaded area were relatively similar, and that the two areas could be compared in their distribution of daily catches for the three species. The three species had a similar distribution of daily catches between the two areas, with a substantial overlap (V. crabro = 65.35%; V. germanica = 40.42%; V. vulgaris = 50.39%). Moreover, abundances of V. crabro and V. vulgaris were higher in the invaded area (mean ± sd, V. crabro = 0.53 ± 0.45; V. vulgaris = 0.02 ± 0.03) than in the area without V. velutina (V. crabro = 0.41 ± 0.50; V. vulgaris = 0.01 ± 0.01) (Fig. 3).
Discussion
This study constitutes a first attempt, for Mediterranean biotopes, to verify whether invasive alien V. velutina and native Vespidae negatively covary in their abundances, as expected in the case of direct competition (especially for V. crabro) or due to a combination of competition and predation (for Vespula spp. species). While we expected native V. crabro to steadily decline with increasing abundances of V. velutina, we found a positive, non-linear, association between the two species, when their numbers were low. Then, at higher abundances, their covariation was weak and characterized by wide credibility intervals. Moreover, when comparing catches between the invaded and the uninvaded areas, we noticed two aspects: (i) abundances of V. crabro were similar between the two areas (and actually higher at the invaded one), and (ii) abundances of V. crabro actually exceeded those of V. velutina, contrary to previous studies from Spain and France (Monceau et al. 2013a; Rodríguez-Flores et al. 2019). Taken together, findings from our statistical model and from our comparison of invaded and uninvaded areas, might indicate a lack of competition between the two species, at least at low abundances. This may be due to the relative recent presence of V. velutina in the invaded area. The conclusion of the lack of competition would align with existing research about direct competition between alien and native species, indicating that competition increases with the number of individuals, due to an increase in the number of inter-specific interactions and a fixed asset of available resources (Ricciardi 2003; Kumschick et al. 2015). The alternative hypothesis would obviously be the lack of competition at any density for a differentiation of the ecological niche of the two species. Concerning invasive alien social wasps, for example, some studies showed that competition with native species was more pronounced at higher abundances (Beggs 2001). Unfortunately, we observed very few trapping sites characterized by high abundances of V. velutina. As a consequence, our model had wide credibility intervals which do not enable us to draw robust conclusions about competition between the two species at high densities. Therefore, we do not exclude that, in contexts where V. velutina is very abundant and can fully exploit its phenology and reproductive traits or in other invaded European areas where the asset of available resources for Vespidae is limited, the competition between the two species could be detrimental for the abundances of V. crabro.
Concerning the relationship between V. velutina and V. crabro, we advance two non-exclusive hypotheses to explain such lack of evident competition effects in the invaded valley. The first one is that niche overlap between the two species is partial, thus V. crabro can escape from competition. Niches between the two hornet species may differ in space, time or food spectrum. Indeed, V. crabro has been indicated as a species well adapted to colonise mountain areas, whereas V. velutina has shown to prefer coastal and low-altitude areas (Bertolino et al. 2016; Monceau and Thiéry 2017; Rodríguez-Flores et al. 2019). The later life cycle of V. crabro compared to the one of V. velutina could be a mechanism that prevents competition through time partitioning (Monceau et al. 2015b). The two hornets have been considered as semi-specialized on honey bees, however they both have shown to be capable of changing food spectrum according to local conditions (Villemant et al. 2011a; Monceau et al. 2013a; Cini et al. 2018; Rome et al. 2021). The second non-exclusive hypothesis, that we advance, is that there is a competition between the two species, although V. velutina is not effectively able to out-compete V. crabro in the invaded area of Italy. The latter species has proved to have a greater fighting ability, linked to its larger body size, which brings V. velutina to avoid direct competition with V. crabro (Kwon and Choi 2020). Vespa mandarinia japonica, which is the biggest Vespidae species as well as the more aggressive in direct fights (Kwon and Choi 2020), is probably acting as an ecological barrier to the spread of V. velutina in Japan (Ikegami et al. 2020). A solid population of V. crabro, operating as an ecological barrier, could be among the reasons that led V. velutina to spread in Italy rather slower compared with the alien hornet expansion in France (Bertolino et al. 2016; Lioy et al. 2019). Finally, also supposing partial trait overlap between the two species, a study has suggested that the presence of V. velutina may even benefit V. crabro as the predation on honey bee colonies weakened by the predation of the alien hornet could facilitate native hornet predation (Monceau et al. 2013b).
In this study, we compared also the abundance of Vespidae species between two close areas of NW Italy. The two areas had similar environmental conditions but differed in the presence of V. velutina. The comparison between invaded/uninvaded areas is an approach widely adopted to detect the effect of biological invasions (Vilà et al. 2010; Kumschick et al. 2015). The distribution of daily catches of V. germanica and V. vulgaris showed a considerable overlap between the two areas, as it was noticed for V. crabro. These results suggest the lack of an evident competition effect that may be due to several not exclusive factors, such as i) a slight differentiation in the niches that helps native wasps to avoid or minimise competition, and ii) V. velutina is not intensively preying on Vespula spp. in our study area, but mainly targeting other insects. Nevertheless, it should be acknowledged that environmental or climatic differences which our sampling design was not able to account for, might have led to the observed overlap of Vespula spp. distributions between the two valleys. Therefore, these results on Vespula spp. are not conclusive, but should be seen as baseline to evaluate long-term impacts due to V. velutina presence.
Our study could be regarded as a first attempt for field validating previous experimental studies, exploring the potential competition between V. velutina and Vespidae species. This study investigates, for the first time to our knowledge, the effect of the invasion of V. velutina over the abundance of native European Vespidae, in a natural environment. V. velutina was included in the European list of invasive species of Union concern, since risk assessment acknowledges the impact of V. velutina upon honey bees (Marris et al. 2011). Nevertheless, a comprehensive evaluation of risk regarding other species was not possible at that time because of the lack of research addressing this issue. This study provides first field-based knowledge on V. velutina impacts on native European wasps. Despite our findings are suggesting a lack of negative effects due to V. velutina, a long-term monitoring programme of wasp populations, based on updated sampling protocols, should be implemented in Europe to detect any potential changes in the interaction with V. velutina, and to provide baseline data for building effective conservation activities.
Data availability
The dataset generated and analysed during the current study is available in the OSF repository, https://osf.io/8c97w/.
Code availability
R’s code generated during the current study is available in the OSF repository, https://osf.io/8c97w/.
References
Arca M, Mougel F, Guillemaud T et al (2015) Reconstructing the invasion and the demographic history of the yellow-legged hornet, Vespa velutina, in Europe. Biol Invasions 17:2357–2371
Bacandritsos N, Papanastasiou I, Saitanis C, Roinioti E (2006) Three non-toxic insect traps useful in trapping wasps enemies of honey bees. Bull Insectol 59:135–145
Barbet-Massin M, Salles JM, Courchamp F (2020) The economic cost of control of the invasive yellow-legged Asian hornet. NeoBiota 55:11–25
Beggs JR (2001) The ecological consequences of social wasps (Vespula spp.) invading an ecosystem that has an abundant carbohydrate resource. Biol Conserv 99:17–28
Beggs JR, Brockerhoff EG, Corley JC et al (2011) Ecological effects and management of invasive alien Vespidae. Biocontrol 56:505–526
Bertolino S, Lioy S, Laurino D et al (2016) Spread of the invasive yellow-legged hornet Vespa velutina (Hymenoptera: Vespidae) in Italy. Appl Entomol Zool 51:589–597
Bessa AS, Carvalho J, Gomes A, Santarém F (2016) Climate and land-use drivers of invasion: predicting the expansion of Vespa velutina nigrithorax into the Iberian Peninsula. Insect Conserv Divers 9:27–37
Buck M, Marshall SA, Cheung DKB (2008) Identification atlas of the Vespidae (Hymenoptera, Aculeata) of the northeastern Nearctic region. Can J Arthropod Identif 5:1–492
Cameron EK, Vilà M, Cabeza M (2016) Global meta-analysis of the impacts of terrestrial invertebrate invaders on species, communities and ecosystems. Glob Ecol Biogeogr 25:596–606
Carbonell JA, Velasco J, Millán A et al (2017) Biological invasion modifies the co-occurrence patterns of insects along a stress gradient. Funct Ecol 31:1957–1968
Choi MB, Martin SJ, Lee JW (2012) Distribution, spread, and impact of the invasive hornet Vespa velutina in South Korea. J Asia Pac Entomol 15:473–477
Cini A, Cappa F, Petrocelli I et al (2018) Competition between the native and the introduced hornets Vespa crabro and Vespa velutina: a comparison of potentially relevant life-history traits. Ecol Entomol 43:351–362
Crowder DW, Snyder WE (2010) Eating their way to the top? Mechanisms underlying the success of invasive insect generalist predators. Biol Invasions 12:2857–2876
Demichelis S, Manino A, Minuto G et al (2014) Social wasp trapping in north west Italy: comparison of different bait-traps and first detection of Vespa velutina. Bull Insectol 67:307–317
Dvořák L, Landolt PJ (2006) Social wasps trapped in the Czech Republic with syrup and fermented fruit and comparison with similar studies (Hymenoptera Vespidae). Bull Insectol 59:115–120
Edwards R (1980) Social wasps. Their biology and control. Rentokil Ltd., Sussex
Franklin DN, Brown MA, Datta S et al (2017) Invasion dynamics of Asian hornet, Vespa velutina (Hymenoptera: Vespidae): a case study of a commune in south-west France. Appl Entomol Zool 52:221–229
Ikegami M, Tsujii K, Ishizuka A et al (2020) Environments, spatial structures, and species competitions: determining the impact of yellow-legged hornets, Vespa velutina, on native wasps and bees on Tsushima Island, Japan. Biol Invasions 22:3131–3143
Islam N, Iftikhar F, Mahmood R (2015) Seasonal variations in hornet’s spp. and efficiency of different traps as a tool for control. Am J Agric Sci 2:223–230
Kumschick S, Gaertner M, Vila M et al (2015) Ecological impacts of alien species: quantification, scope, caveats, and recommendations. Bioscience 65:55–63
Kwon O, Choi MB (2020) Interspecific hierarchies from aggressiveness and body size among the invasive alien hornet, Vespa velutina nigrithorax, and five native hornets in South Korea. PLoS ONE 15:e0226934
Laurino D, Lioy S, Carisio L et al (2020) Vespa velutina: an alien driver of honey bee colony losses. Diversity 12:5
Lemoine NP (2019) Moving beyond noninformative priors: why and how to choose weakly informative priors in Bayesian analyses. Oikos 128:912–928
Lioy S, Manino A, Porporato M et al (2019) Establishing surveillance areas for tackling the invasion of Vespa velutina in outbreaks and over the border of its expanding range. NeoBiota 46:51–69
Lioy S, Laurino D, Capello M et al (2020) Effectiveness and selectiveness of traps and baits for catching the invasive hornet Vespa velutina. Insects 11:706
Marris G, Brown MA, Cuthbertson AG (2011) GB non-native organism risk assessment for Vespa velutina nigrithorax. Available at www.nonnativespecies.org. Accessed on 3 February 2021
Mckinney ML, Lockwood JL (1999) Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends Ecol Evol 14:450–453
Moller H (1996) Lessons for invasion theory from social insects. Biol Conserv 78:125–142
Monceau K, Thiéry D (2017) Vespa velutina nest distribution at a local scale: an 8-year survey of the invasive honeybee predator. Insect Sci 24:663–674
Monceau K, Maher N, Bonnard O, Thiéry D (2013a) Predation pressure dynamics study of the recently introduced honeybee killer Vespa velutina: learning from the enemy. Apidologie 44:209–221
Monceau K, Arca M, Leprêtre L, Mougel F, Bonnard O, Silvain JF, Maher N, Arnold G, Thiéry D (2013b) Native prey and invasive predator patterns of foraging activity: the case of the yellow-legged hornet predation at European honeybee hives. PLoS ONE 8:e66492
Monceau K, Bonnard O, Thiéry D (2014) Vespa velutina: a new invasive predator of honeybees in Europe. J Pest Sci 87:1–16
Monceau K, Moreau J, Poidatz J et al (2015a) Behavioral syndrome in a native and an invasive hymenoptera species. Insect Sci 22:541–548
Monceau K, Maher N, Bonnard O, Thiéry D (2015b) Evaluation of competition between a native and an invasive hornet species: do seasonal phenologies overlap? Bull Entomol Res 105:462–469
Pearl J (1995) Causal diagrams for empirical research. Biometrika 82:669–688
Pearl J, Mackenzie D (2018) The book of why: the new science of cause and effect. Basic Books, New York
Poidatz J, Bressac C, Bonnard O, Thiéry D (2018) Comparison of reproductive traits of foundresses in a native and an invasive hornet in Europe. J Insect Physiol 109:93–99
Reitz SR, Trumble JT (2002) Competitive displacement among insects and arachnids. Annu Rev Entomol 47:435–465
Requier F, Rome Q, Chiron G et al (2019) Predation of the invasive Asian hornet affects foraging activity and survival probability of honey bees in Western Europe. J Pest Sci 92:567–578
Ricciardi A (2003) Predicting the impacts of an introduced species from its invasion history: an empirical approach applied to zebra mussel invasions. Freshw Biol 48:972–981
Robinet C, Darrouzet E, Suppo C (2019) Spread modelling: a suitable tool to explore the role of human-mediated dispersal in the range expansion of the yellow-legged hornet in Europe. Int J Pest Manag 65:258–267
Rodríguez-Flores MS, Seijo-Rodríguez A, Escuredo O, del Seijo-Coello M C (2019) Spreading of Vespa velutina in northwestern Spain: influence of elevation and meteorological factors and effect of bait trapping on target and non-target living organisms. J Pest Sci 92:557–565
Rojas-Nossa SV, Novoa N, Serrano A, Calviño-Cancela M (2018) Performance of baited traps used as control tools for the invasive hornet Vespa velutina and their impact on non-target insects. Apidologie 49:872–885
Rome Q, Perrard A, Muller F et al (2021) Not just honeybees: predatory habits of Vespa velutina (Hymenoptera: Vespidae) in France. Ann Soc Entomol Fr 57:1–11
Säterberg T, Sellman S, Ebenman B (2013) High frequency of functional extinctions in ecological networks. Nature 499:468–470
Schielzeth H (2010) Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol 1:103–113
Seebens H, Blackburn TM, Dyer EE et al (2017) No saturation in the accumulation of alien species worldwide. Nat Commun 8:14435
Simberloff D, Martin JL, Genovesi P et al (2013) Impacts of biological invasions: what’s what and the way forward. Trends Ecol Evol 28:58–66
Snyder WE, Evans EW (2006) Ecological effects of invasive arthropod generalist predators. Annu Rev Ecol Evol Syst 37:95–122
Sorvari J (2013) Social wasp (Hymenoptera: Vespidae) beer trapping in Finland 2008–2012: a German surprise. Entomol Fenn 24:156–164
Spradbery JP (1973) Wasps. An account of the biology and natural history of social and solitary wasps, with particular reference to those of the British Isles. Sidgwick and Jackson, London
Stoett P, Roy HE, Pauchard A (2019) Invasive alien species and planetary and global health policy. Lancet Planet Heal 3:e400–e401
Strauss A, White A, Boots M (2012) Invading with biological weapons: the importance of disease-mediated invasions. Funct Ecol 26:1249–1261
Verdasca MJ, Godinho R, Rocha RG, Portocarrero M, Carvalheiro LG, Rebelo R, Rebelo H (2021) A metabarcoding tool to detect predation of the honeybee Apis mellifera and other wild insects by the invasive Vespa velutina. J Pest Sci 95:997–1007
Vilà M, Basnou C, Pyšek P et al (2010) How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross-taxa assessment. Front Ecol Environ 8:135–144
Villemant C, Muller F, Haubois S (2011a) Bilan des travaux (MNHN et IRBI) sur l’invasion en France de Vespa velutina, le frelon asiatique prédateur d’abeilles. In: Barbançon JM, l’Hostis M (eds) Proceedings of the Journée Scientifique Apicole, Arles. pp 3–12. Accessed on 11 Feb 2011
Villemant C, Barbet-Massin M, Perrard A et al (2011b) Predicting the invasion risk by the alien bee-hawking Yellow-legged hornet Vespa velutina nigrithorax across Europe and other continents with niche models. Biol Conserv 144:2142–2150
Acknowledgements
We express special thanks to Mattia Bessone for his initial effort in planning the activities for the realization of this study and for setting up the sampling stations. We acknowledge Andrea Romano, Michela Capello, Alessandro Viscardi, Marco Gallesi and Paolo Dal Col for their valuable support in field activities. We thank ‘Birra 100 Venti’ for providing us with the bait for carrying out the monitoring activity. We also thank the referees for their valuable suggestions in improving the manuscript.
Funding
Open access funding provided by Università degli Studi di Torino within the CRUI-CARE Agreement. This work was realised with the contribution of the EU funded project LIFE14 NAT/IT/001128 STOPVESPA of the LIFE Programme.
Author information
Authors and Affiliations
Contributions
Conceptualization, SL, SB, MP; Data curation, LC, JC, SL; Formal Analysis, LC, JC; Funding acquisition, SB, MP; Investigation, LC, EB; Fieldwork, EB; Project administration, SL, MP; Supervision, SB, MP; Visualization, LC, JC; Writing—original draft, LC, JC; Writing – review & editing, LC, JC, SL, EB, SB, MP.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare that are relevant to the content of this article.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carisio, L., Cerri, J., Lioy, S. et al. Impacts of the invasive hornet Vespa velutina on native wasp species: a first effort to understand population-level effects in an invaded area of Europe. J Insect Conserv 26, 663–671 (2022). https://doi.org/10.1007/s10841-022-00405-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-022-00405-3