Abstract
Anthophora retusa is a rare solitary bee which has declined throughout Britain and other European countries since the 1990s. It is thought to be restricted to five sites in Britain. However, information on these remaining populations is limited. Knowledge on population size, habitat and forage requirements and foraging distance, are important for successful conservation of species. The population of A. retusa at the Seaford Head Nature reserve in East Sussex was surveyed. Transects within the reserve were conducted and population estimates using mark recapture were made for 2018 and 2019. Pollen from foraging females was analysed alongside visual sightings to determine forage requirements. The total population was estimated to be 91 in 2018 (males and females) with an estimated male population of 167 in 2019. The most visited flower species by females was Glechoma hederacea (66% of visits) but flower preference changed throughout the flight season, shifting to Fabaceae species and Iris foetidissima with 16 plant groups identified in pollen samples. Bees were geographically restricted to a small area within the reserve (approximately 30 ha). Although the exact location of nesting sites was not determined with certainty it is thought nests are in the loess deposits at the top of the inaccessible sea cliff face. This project suggests the presence of appropriate nesting sites may be limiting A. retusa distribution as they appear to forage on common plant species. More research is needed on the exact nesting requirements of the species.
Implications for Insect Conservation
The findings from this paper help contribute to the limited understanding of the ecology of this rare and declining species. By knowing the forage requirements of A. retusa, other areas where it is found can ensure these are present within a short distance of nest sites, hopefully ensuring the survival of individual populations and therefore the species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the ecology of a rare species is vital in devising appropriate conservation strategies. Global examples of where this understanding has resulted in conservation success stories include the Asiatic lion in the Gir forest (Singh and Gibson 2011) and mountain gorillas in Bwindi national park (McNeilage et al. 2006). Examples in the UK include the Large Blue butterfly (Thomas 1995) and the Eurasian bittern (Brown et al. 2012).
In addition, information on population sizes and trends is required to determine if a species is rare, or if a population is small and at risk of extinction (Jackon 2019). Understanding these trends is becoming increasingly important in the current ecological climate with declines reported in many species globally (Thomas et al. 2004; Beebee and Griffiths 2005; Goulson et al. 2008; Winfree et al. 2009; Potts et al. 2010). Species extinctions begin with the loss of local populations. Smaller, isolated populations are at increased extinction risk (Purvis et al. 2000), especially in species with intermediate mobility (Thomas 2000; Traill et al. 2007) or that have specific habitat or resource requirements (Thomas 1995).
In the last 600 years 70 insect species have been declared extinct globally (Dunn 2005). However, this number is thought to be much less than what is expected due to lack of research on certain insect groups, with one proposed estimate of 44,000 species extinctions (Dunn 2005). Globally, pollinator species are in decline (Biesmeijer et al. 2006; Potts et al. 2010). In Europe, 2.4% of bee species are classed as critically endangered by the IUCN (Nieto et al. 2014). However, again due to limited monitoring, this figure is an estimate, as for 57% of European species there is not enough data to evaluate their extinction risk (Nieto et al. 2014). An example of a bee species thought to have recently gone globally extinct is Bombus franklini. It had a narrow distribution, only ever recorded in an area approximately 240 by 110 km in southern Oregon and northern California in the United States (National Research Council 2007). For bees, the threat of extinction is not just limited to B. franklini, with four other Bombus species in Northern United States being placed on the IUCN at risk list for pollinators (National Research Council 2007).
Globally there are an estimated 20,000 species of bee. Of these, 250 are bumblebees (Goulson et al. 2008), approximately 300 are stingless bees (de Oliveira Francisco and Arias 2010) and nine are honeybees (Koeniger and Koeniger 2000). Most of the remaining c. 19,500 are not eusocial and are often called solitary bees. There are many studies examining bumblebee species declines (Goulson et al. 2008; Cameron et al. 2011; Meeus et al. 2011) in the UK and elsewhere. However, studies on solitary bee species are minimal. This is shown with a Web of Science© search for bumblebees in the title or topic returning 1332 studies between 2010 and 2020, with the same search for solitary bees returning only 411.
In Britain 12 species of bee have gone extinct since 1851, of which 2 were bumblebees, although no extinctions have been reported since 1990 (Ollerton et al. 2014). However, from 1980 to 2013 British solitary bees have suffered a 32% decline in average occupancy (Powney et al. 2019). Declines of both bumblebees and solitary bees in the UK are thought to be due to a variety of factors including habitat destruction, intensification of agriculture (Buchmann and Ahrne 2005), reduced growing of clover due to the invention and use of nitrogenous fertilizers (Ollerton et al. 2014) and pesticides (Goulson et al. 2015; Woodcock et al. 2017).
Solitary bees are not only an important component of biodiversity, but also contribute to pollination of both crops (Klein et al. 2003; Ricketts et al. 2004; Greenleaf and Kremen 2006; Holzschuh et al. 2012) and wild plant species (Ollerton et al. 2011; Rollin et al. 2013). Solitary bees are at risk of local extinctions due to their often-limited foraging distances (Gathmann and Tscharntke 2002; Greenleaf et al. 2007), specialised nesting (Westrich 1996; Wcislo and Cane 1996; Zurbuchen et al. 2010) and forage requirements (Westrich 1996; Wood et al. 2017). Determining these requirements, such as plant species on which they forage and where they nest, are likely to be important in making habitat management effective.
Anthophora retusa, the flower potter bee, is a spring- flying (active from April to late June), large (forewing length 8.5–10 mm, Falk and Lewington 2015) solitary bee. It used to be widespread in southern England but since the 1990s it has greatly declined and is now restricted to a few sites (Table 1). It is thought that the species has become regionally extinct in eastern England as the last records from previously reported sites in Norfolk and Essex were in the early 1970s (Jackson 2019). The sites where it is still found are geographically small (Table 1). For example, in Dorset A. retusa are found nesting and foraging in a quarry site of approximately 30 ha.
This decline of A. retusa is thought to be due to the intensification of landscape for farming and loss of nesting habitat as well as threats from flooding and cliff erosion (Evans and Potts 2004). It is a UK BAP priority species and is listed as Endangered (Falk 1991). Although assigned as Least Concern at the European level by Nieto et al. in 2014, due to it being widespread and common in the south of its range, the species has also been red listed in eight out of the 20 countries where it is found. Within these eight it is regarded as critically endangered in Estonia and endangered in the Czech Republic and the Netherlands, but there is very little information on individual populations. The declines mirror that of some bumblebee species (Edwards and Jenner 2008).
A geographically restricted population of A. retusa was identified at the Seaford Head local nature reserve in East Sussex in the 1990s by Edwards and Jenner (2008). The aims of this study were to determine several important ecological parameters for this population. First, population size using mark recapture. Second, to determine their main sources of forage using both pollen analysis and observations of individual foragers. And third, its foraging range within the study area at Seaford Head using transect surveying of bees on flowers. In addition, this information was used to estimate foraging locations and distances travelled when foraging.
Methods
Study site
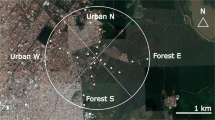
Seaford Head Local Nature Reserve is located east of Seaford town and is bordered to the south by chalk sea cliffs and to the east by the Cuckmere River estuary. It is within the Sussex Downs Area of Outstanding National Beauty and is jointly owned by Seaford Town Council, the National Trust and the East Sussex County Council. The landscape consists of coastal scrub, pasture grazed by sheep and cattle and both grazed and ungrazed grassland rich in wildflowers. It is classed as a nature reserve and SSSI (Site of Special Scientific Interest. Natural England 1999) due to the presence of nationally rare species, including Anthophora retusa, Adscita statices (forester moth) and Seseli libanotis (moon carrot) (Sussex Wildlife Trust). There is a diversity of habitat types throughout the reserve. The section of the reserve where the survey was conducted consists of a mixture of scrubland and occasionally grazed grassland bordered by permanent pasture (Fig. 1a). It is the only part of the reserve where the cliffs have exposed loess deposits, highly porous silt sediment formed by the accumulation of windblown dust (Frechen 2011). The western section is predominately scrubland bordered by the Seaford golf course (Fig. 1b). The eastern side is low-lying meadow with the Cuckmere river flowing through the centre (Fig. 1c).
The total survey area (Fig. 1a) was 25 ha, approximately 30% of the 83 ha reserve. Within this, land was classified into three habitat types (scrubland, cliff edge and grassland) and 11 fixed survey-transect routes were established (Supplementary Fig. 1). The survey area was chosen as a previous study by Edwards and Jenner (2008) and surveys conducted by Sussex Wildlife had identified the presence of A. retusa along the exposed cliff face and inland within the 25 ha survey site. The site also ensured multiple habitat types could be included in the survey.
Areas were classified as scrubland where species such as Crataegus monogyna (hawthorn), Sambucus nigra (elder) and Rubus fruticosus (bramble) were dominant and accounted for approximately 10 ha with four transect walks (Fig. 1a). Grassland was defined as areas with minimal or no tree cover, more than 100 m from the cliff edge with wild flowers or low (less than 1.5 m high e.g. Gorse) shrubs as the predominant vegetation. This accounted for approximately 10 ha and contained five transects. The remaining 5 ha was classified as cliff edge with wild flowers and grass the predominant vegetation and was any area than fell within 100 m of the cliff edge and contained two transects (Fig. 1a). Transects were made along pre-existing paths with adjacent flower patches being surveyed.
Mark-recapture and transect-survey walks
Mark -recapture data were collected 4 May to 18 June 2018 and 15 April to 22 June 2019 as these dates covered the period of peak activity for A. retusa (Falk and Lewington 2015). Surveys were adjusted to start earlier in 2019 to increase the survey time when males were active. Surveys were ideally made every two or three days but due to weather conditions not conductive to bee activity, sunny and 12 °C or more, this was not always possible. The same transect routes were walked each survey day between 10:00 and 15:00 with the order randomised each day to minimise both time of day and weather effects on bee activity. Routes were walked once per survey day at a consistent speed with the same observer each day.
One challenge in studying A. retusa is that it cannot be differentiated on the wing from the very similar looking and abundant A. plumipes (Fig. 2a, b). Therefore, whenever either were seen they were caught with an insect net and identified. Females were identified by the colour of the hind tibial spur. In A. retusa this is red (Fig. 2a) versus black in A. plumipes. Males of the two species could be identified because only A. plumipes has the obvious longer hairs on the mid tarsi (Fig. 2c). Following species identification, A. plumipes were released. Any A. retusa were released after being marked on the notum with individual colour-dot combinations using Revell© water based acrylic paints (Fig. 2d, Zurbuchen et al. 2010).
a Female Anthophora. retusa with arrow indicating the red hind tibial spur (Aubert 2013) b Female Anthophora. plumipes resting on a leaf (Jones 2013) c Male A. plumipes with the distinctive hairy feet (Owens 2011) which does not occur in in A. retusa d Paint-dot-marked male A. retusa foraging on ground ivy, Glechoma hederacea
In addition, the flower species each bee was foraging on plus the GPS coordinates were recorded. If the bee was female, we attempted to collect a pollen sample. This was done by placing the female in a queen marking cage and using a fine toothpick to scrape grains from the scopa and the whole body into individual Eppendorf tubes which were frozen until analysis. Due to the rarity of the species, destructive sampling to ensure all pollen was collected from each female was not appropriate. Care was taken to remove all pollen from a female with the same technique of collection, as explained above, for each bee even if pollen did not appear to be present, to minimise the risk of only collecting larger or more visible grains. Occasionally pollen was not obtained from captured females either due to too few grains present or because the female was not collecting pollen. As a result, pollen samples were not collected on every survey day.
Using the GPS coordinates, the mean travel distances between the initial capture location and recapture points were calculated (Peakall and Schiestl 2004). If multiple recaptures of the same individual occurred (5 individuals in 2019 and 3 in 2018) then the mean distance travelled was calculated using the distances from the first capture point to all subsequent capture locations. In 2019 a large area of kidney vetch, Anthyllis vulneraria, was located along the cliff face. On 31 June, individuals foraging on this patch were caught using a net with a telescopic handle, marked and pollen samples taken from females. This was the only day where the patch was surveyed as it had not been previously identified and, therefore, to maintain a constant survey effort was not added to the survey route. To determine if female A. retusa had habitat preferences the average number of captures between the different habitat areas and transects were compared.
Additional surveys were conducted outside of the core 25 ha study area to determine if A. retusa was present in a nearby location where it has previously been found (Falk pers. Comms) and areas adjacent to the study site. On 28 May 2018, a survey was conducted inland from Seaford Head through the Cuckmere valley for approximately 4.5 km, ending at coordinates 50.791003, 0.156665 (Supplementary Fig. 2a). In 2020, additional surveys within Seaford head reserve were completed. These occurred in the western side which contains Seaford golf course (Fig. 1b) and the surrounding wetlands to the east on both sides of the river (Fig. 1c) along with the original 25 ha survey area (Fig. 1a). Outside of the reserve the wetlands on the east side of the river (Fig. 1d) ending at coordinates 50.774232, 0.152147 and an area where A. retusa were once recorded, High and Over (coordinates 50.789516, 0.140185, Edwards per comms., Supplementary Fig. 2b) were also surveyed.
Each area was visited a total of three times, on days of good weather (above 16 °C and dry) between May 27 and June 8 2020 during the female foraging period. Mark recapture and pollen collection were not conducted during these additional surveys.
Surveys additional to our study were made by Graeme Lyons of the Sussex Wildlife Trust in the western side of the nature reserve in 2016 and 2018 (Fig. 1b) and inland along the Cuckmere river for approximately 2 km (2018, Fig. 1c).
Population size models and estimates
To estimate population sizes for males and females in both 2018 and 2019 the POPAN model of the Jolly–Seber method for open populations (Schwarz and Arnason 1996) was used in the programme “Mark” (version 6.2). Separate models were made for males and females for both years as previous work suggests separate models for sexes gives more accurate predictions (McKnight and Ligon 2017). Multiple models with different parameter criteria were created. The parameter index matrices (PIMs) used in the POPAN method include; Ф (the apparent survival rate), p (capture probability), pent (probability of entry into the population) and N (super-population size). Each of these PIMs was tested as either time dependent or constant and the “Akaike information criterion” (AIC) was used to select the most suitable model. Goodness of fit testing was performed to ensure the data met the model assumptions.
Pollen identification
Pollen was mounted in glycerine jelly with basic fuchsin to stain individual grains (Wood et al. 2018). Identification was under light microscope at 400x magnification. Due to the low number of pollen grains present in each sample it was possible to count the actual number of grains per plant species identified. Identification to species level was attempted. However, due to similarities among species within Trifolium, other Fabaceae and Apiaceae these were identified to either genus or family level. Samples were grouped by date collected. The percentage of pollen grains from each plant species was calculated from the total number of grains identified on each sample day. If multiple bees were sampled on a day the data were pooled.
Flower-abundance transects
In 2018 the abundance of flower species on each transect was recorded using a modified DAFOR vegetation scale (Croxton et al. 2005). For each survey day the species present on each transect and its abundance on a scale of 0 to five (0: absent; 1: rare; 2: occasional; 3: frequent; 4: abundant and 5: dominant). The overall daily abundance for each species was calculated as the average of its abundance across all transects for that day. The average abundance during the flowering period was calculated only using dates where the species was seen to be in flower on at least one transect. These measures provide information regarding when certain species became abundant within the female flight period and which species were flowering consistently throughout. Average abundance during the survey period was calculated using the average daily abundance for all survey dates. The flower survey dates were 20 and 30 May and 5,11 and 18 June 2018 to overlap with female activity. To determine differences in plant abundances between habitats, the average abundance of the five most visited plant groups by A. retusa females were compared between habitat areas and transects. These groups either accounted for more than 20% of flower visits or were over 20% of the pollen grains identified.
Statistical analysis
To determine if there was a significant difference in when peak activity occurred between males and females Mann-Whitney U tests were completed for both years separately due the difference in survey start date between years. To test for differences in plant abundance and the number of females captures between habitat types and transects Kruskall Wallis tests were run. To test for differences between survey years and sexes in distances between capture points t-tests were performed. All statistics were performed using R version 3.4.2.
Results
Population estimates
In 2018 50 males and 42 females were caught and marked on 13 survey days, from 4 May to 18 June (Supplementary table 1) No males were caught after 6 June. The day with the most male captures was 5 May, with 10, and 13 June for females, with 12 captures.
In 2019 there were 19 survey days from 4 May to 13 June. A total of 160 males and 57 females were caught (Supplementary table 2). As in 2018, the day with the highest number of male captures was earlier than for females (10 May vs. 6 June).
In both 2018 and 2019 males were on the wing significantly earlier than females (Mann Whitney test U, 2-tailed: 2018, U = 1874.5, P < 0.0001; 2019, U = 6777, P < 0.0001).
To obtain population estimates separate models were run for each year and sex. Using goodness of fit testing, models that best fitted the data were chosen. The models used are shown in supplementary table 3. The estimated ratio of males to females in 2018 was 1.1:1.0 In 2019 the male population was estimated to be approximately 3.6 times greater than in 2018, increasing from 46.8 to 167.1 individuals (Table 2).
a Male and female daily population estimates for 2018; b Male daily population estimates for both 2018 and 2019. Standard error bars are shown. Horizontal lines indicate the overall mean of the daily population estimates. There were no daily population estimates for females in 2019 because zero recapture occurred
The daily population average for females in 2018 was 5.7 individuals and for males was 7.7 (Fig. 3a).
In 2019 the male population daily maximum was 71% higher than 2018 and the daily minimum was 38% higher (Fig. 3b). The daily population average for males in 2019 was 17.3.
Pollen on females
In 2018 pollen was collected from 14 foraging females across the survey period. Pollen from 12 different plant species or families was identified. In 2019 pollen was collected from 12 foraging females with pollen from 14 different species or families identified. Pollen from ground ivy, Glechoma hederacea and Fabaceae species were the most common for both years. Glechoma hederacea occurred in 64.3% (9/14) of samples and Fabaceae species in 50% (7/14) in 2018. In 2019 G. hederacea was present in 66.7% (8/14) and Fabaceae species in 75% of samples (9/12, Table 3). Nearly all females had pollen from at least two species or families (26/27) and pollen was often identified from plant species that A. retusa females were not seen foraging from, such as hawthorn (Crataegus monogyna). On average females were carrying pollen from 2.9 species.
In 2018 the four most prevalent plant species identified in pollen samples were C. monogyna, G. hederacea, Echium vulgare (viper’s bugloss) and species of Fabaceae (excluding Trifolium sp.) (Fig. 4a). Glechoma hederacea was present in five out of the seven days sampled and species of Fabaceae were present in six. The date when plant species other than the top four most common were more prevalent was 30 May, with only 20% of pollen being from the Fabaceae and the remaining 80% from Iris foetidissima (stinking iris) and Apiaceae species (Fig. 4a).
In 2019 the three most prevalent plant species identified in pollen samples were G. hederacea and species of Fabaceae and Trifolium (T. pratense and T. repens) (Fig. 4b) G. hederacea pollen was the most common being present in samples from six of the seven days. Other plant species were most common on 22 June with E. vulgare comprising 78% of the samples and Ranunculus sp. comprising 7% (Fig. 4b).
When pooled over all sample days in 2018 G. hederacea pollen grains comprised most of the pollen (33%) followed by E. vulgare (15%), C. monogyna. (13%) and Fabaceae species (12%, Fig. 4c). In 2019 G. hederacea pollen was the most common (34%) followed by Trifolium species (33%) and then Fabaceae species (22%).
Survey walks
On 2018 survey walks most females were seen foraging on G. hederacea (63%) followed by I. foetidissima (29%, Fig. 5c). On seven of the nine survey days all females seen were foraging on G. hederacea. Females were only seen foraging on I. foetidissima from 6 June (Fig. 5a). Data from 2019 females followed a similar pattern, with most females seen foraging on G. hederacea (69%), followed by I. foetidissima (20%). All visits to A. vulneraria were recorded on 31 May. This was due to a large abundance of A. vulneraria on the cliff face being accessed and 0 females seen elsewhere in the study area on this day. A. vulneraria was not found elsewhere in the survey area nor was it located in the 2018 surveys. Females were first seen visiting I. foetidissima on the same date as the previous year (6 June, Fig. 5b).
In 2020 an additional 47.1 km of survey walks were made outside the core study area. On the western side of the Seaford Head reserve (Fig. 1c) 15.4 km were walked in 4 h with zero A. retusa seen. On the eastern side of the Cuckmere river, in the area of wetlands not within the Seaford Head reserve (Fig. 1d), 16.8 km were walked in 4 hours also with zero A. retusa seen. In the wetlands located on the western side of the river within the reserve (Fig. 1c) 7.4 km were walked in 2 h. A total of 24 female A. retusa were found within this area all foraging either on G. hederaceae (12) or E. vulgare (12). The furthest distance A. retusa was found outside of the initial study area was 250 m. Beyond this zero A. retusa were located. Surveys within the core area in 2020 covered 6 km in 4 h with 26 female A. retusa identified. All were foraging on I. foetidissma. Surveys further inland at High and Over, a site where A. retusa have historically been seen, covered 6.6 km in 2 h of surveying with no A. retusa identified.
Flower abundance
A total of 59 plant species were identified in bloom in the survey area (Table 4). The average abundances and flowering periods for the five most visited plant species, either through observation or pollen samples, are in Table 4. Of these G. hederacea was most abundant (mean abundance score 2.4). When including all flower species the most abundant on average across the survey period was Ranunculus sp. (Buttercups) (2.5). Although in flower throughout the entire survey period the average abundance of G. hederecae decreased each survey week, with its peak abundance score of 3.6 (on the first week of floral surveys, 20 May) declining to 1.4 on the last survey week (18 June, Supplementary Fig. 3). I. foetidissima, the second most visited plant by female A. retusa ,followed the opposite trend of not being in flower at the beginning of the survey period (Supplementary Fig. 3) and reaching peak abundance on the only transect where it was found (score of 5) on 11 June.
Trifolium species (Kruskall–Wallis, χ2 = 6.2, df = 2, P = 0.05) and I. foetidissima (Kruskall-Wallis, χ2 = 11.3, df = 2, P = 0.003) differed in abundance between habitat types. A post hoc Dunn’s test showed that Trifolium sp. were significantly more abundant on the coastal transects compared to those in the scrub habitat (P = 0.02) with no difference in abundance between grassland and coastal habitats (P = 0.06). I. foetidissima was only identified along a single transect in the scrub habitat. When comparing species abundance between transects I. foetidissima and Fabaceae abundance differed significantly (I. foetidissima; Kruskall–Wallis, χ2 = 41.7, df = 10, P < 0.001, Fabaceae; χ2 = 29.1, df = 10, P = 0.001) but none of the other plant species tested did (Supplementary table 5).
Spatial distribution
Given the low numbers of recaptures in 2018, zero recapture of females in 2019 (Supplementary table 2 and 3) and a non-significant difference in distances between years (t = − 0.9, p = 0.19) and sexes (t = − 1.3, p = 0.11), distance data were pooled for analysis. The average distance between the initial capture and subsequent recaptures was 122 ± 21.2 m (Fig. 6) with most observations less than the mean (range 0–486 m, mode 0–40 m). When an individual had multiple recaptures, the mean distance travelled was calculated using the distances from the first capture point to all subsequent capture locations.
The GPS locations of caught individuals indicate foraging hotspots, especially for females (Supplementary Fig. 1). Clusters of females are in areas of either high G. hederacea density, or later in the season, I. foetidissima. In Supplementary Fig. 1 the black rectangle indicates the transect (A1T3) where over the two years 43% of female captures occurred. This area accounts for only 5% of the whole study site with the average abundance of G. hederacea along the transect route being 3 (frequent) in 2018. It was the only transect where I. foetidissima was present, with an average abundance of 3.3 during its flowering period in 2018. There was a significant difference in the number of female captures between transects for both years (2018; Kruskall–Wallis, χ2 = 36.3, df = 10, P < 0.001, 2019; Kruskall-Wallis, χ2 = 27.5, df = 10, P = 0.002). A post hoc Dunn’s test showed that transect A1T3 had significantly more female captures compared to all other transects for both years (Supplementary table 4).
However, when examining capture rates between habitat types (scrub, grassland and coastal) there was no significant difference in 2018 (Kruskall–Wallis, χ2 = 0.4, df = 2, P = 0.81) or 2019 (ANOVA, F2,8 = 2.92, P = 0.11).
A potential nesting area was identified in 2019 as males were seen patrolling along the cliff face and a mating pair was found a few meters from the edge. Due to the inaccessibility of the cliff face for safety reasons no individuals could be identified. However, females were seen flying up from the cliff face and small excavations, possibly nests, could be seen in the layers of loess deposits at the cliff edge.
Discussion
Our results indicate that the A. retusa population at Seaford Head is very small and geographically confined. Mark-recapture indicates that the male population in 2019 (the last year of population surveying) was less than 200 individuals, with bees not seen outside of the Seaford Head reserve, including in surveys conducted in 2020. The small size of the population is unlikely to be because A. retusa forages on rare plants or specialises on a narrow range of species, as is the case in some rare bees (Zayed and Packer 2007) and insect herbivores (Ellis et al. 2019). The plant species that was predominantly used for foraging was ground ivy, G. hederacea, which is extremely abundant in the local area and nationally. One important missing piece of information in our study is that we were unable to locate and study nest sites. However, it is likely that nesting was in windblown, loess deposits of soil and sand on top of the chalk, which is vertically exposed at the cliff face (Edwards and Jenner 2008; Horsley et al. 2013). This deposit is an unusual feature of the cliff at Seaford Head and is not found, for example, on top of the chalk cliffs of the Seven Sisters and Beachy head, which run to the east of Seaford Head (Sussex Wildlife Trust).
Small isolated populations have a high risk of local extinction due to a wide range of factors including habitat loss, weather, demographic stochasticity and genetic influences (Shaffer 1981; Lande 1993). Coastal erosion is an accelerating process at Seaford Head, with an increase from 2 to 6 cm year− 1 from the Holocene to 22–32 cm year− 1 in the last 150 years (Hurst et al. 2016). Frequent cliff falls may threaten nesting sites if these occur on the cliff face, for example in 2017 50,000 tonnes of cliff fell in one location along the edge (BBC 2017). A fall that large could result in a big proportion of nests being destroyed, particularly if they are aggregated. Although major cliff falls are a threat to nesting aggregations, erosion is a natural and important process for cliff nesting species (Evans and Potts 2004).
Male population within our survey period reached its peak approximately a month earlier than the female peak, indicating that A. retusa emergence is protandrous, a common characteristic of solitary bee species and many other insects (Eickwort and Ginsberg 1980). Mark-recapture showed a 255% increase in the estimated gross male population from 2018 to 2019. This may have been in part due to a longer survey period in 2019, which began earlier and therefore encompassed more of the male’s flight period. However, the daily population estimates also increased, more than doubling, from an average of 7.7 in 2018 to 17.3 in 2019. The change in female population between the two years is unknown because zero females were recaptured in 2019. The reason for this is not known. It might be due to the paint marker used rubbing off the females as, unlike males, they are involved in nest excavation, increasing this chance of removal. However, the same paint brand was used for both years and is used in other mark recapture studies (Zurbuchen et al. 2010). Another explanation could be that, later in the flight season, females were restricting their foraging activity to a large patch of A. vulneraria located along the cliff face. Multiple females were seen foraging here and were inaccessible for safety reasons. Therefore, if marked it was not possible to recapture them. These flowers were not discovered in 2018, therefore it isn’t known if females were foraging on them to the same extent. A final explanation could be the population is much larger than expected. However, the sex ratio in 2018 was approximately even, 1.1:1.0F, which is expected on evolutionary grounds (Fisher 1930). Therefore, a much larger female population compared to the male population in 2019 is unlikely.
Sufficient and appropriate forage is vital for population survival and persistence (Schultz and Dlugosch 1999). G. hederacea was the major floral resource for female A. retusa, especially at the beginning of their flight season. Over both years a total of 16 different plant species and families were identified in pollen samples, with G. hederacea, E. vulgare and Fabaceae species being the most common, indicating that A. retusa is polylectic (Mueller and Kuhlmann 2008). In 2018 G. hederacea was the most prevalent pollen (was 34% of all pollen grains identified) and in both years was the most popular, representing 73% of all flower visits recorded during transects (Fig. 5). The second most visited plant species was stinking iris, I. foetidissima (23% of flower visits). However, pollen from I. foetidissima was only found in 12% of samples (3/26 bees) and comprised less than 1% of the total grains identified across both years. This perhaps suggests that I. foetidissima is more important as a source of nectar than pollen. No males were seen foraging on I. foetidissima. However, there were very few males still foraging during the flowering period of this plant species. Female foraging preference was flexible, following changes in flower abundance, primarily the reduction of G. hederacea and bloom of I. foetidissima. The lack of visual sightings on some of the plant species identified in pollen samples may be due to inaccessibility of certain parts of the survey site, with A. retusa foraging on these species in places where surveying was not possible (such as through dense scrub or on the very edge of the cliff face). These results indicate that the presence of particular flower species may not be as important as forage availability from a succession of species across the flight season.
The location of forage may also be important. Although the number of female captures did not differ between habitat types, one transect, in the scrub habitat, did have significantly more female captures than all others (Supplementary Fig. 1). Surrounding vegetation sheltered this transect from the wind. In addition, it contained abundant G. hederacea and later in the season was the only transect where I. foetidissima was found. Flower abundance has previously been found to influence bee foraging patterns (Goulson 1999; Hegland and Boeke 2006). Only I. foetidissima and Fabaceae species abundance differed between transects, with I. foetidissima abundance also differing on the larger scale of habitat type, along with Trifolium species. This lack of a larger scale differences in flower abundance of the most visited plant species by A. retusa may explain why the number of female captures did not differ between habitats, as the floral composition was relatively similar throughout the survey site. The higher number of captures on the single transect with the presence of I. foetidissima may indicate its importance as a floral resource.
These hotspots of A. retusa activity may also be due to individuals travelling only short distances to forage and seek mates. Most individuals (male and female) were caught less than 30 m away from their initial capture. Being a large bee species (forewing length 9–10 mm for females and 8.5–9 mm for males, Falk and Lewington 2016) A. retusa should have the ability of foraging at moderate to large maximum distances (c. 700 m or more), given that foraging distance correlates with body size (Gathmann and Tscharntke 2002; Greenleaf et al. 2007).
Hofmann et al. (2020) found significantly larger maximum flight distances compared to observed average flight distances in six solitary bee species, with maximum estimates up to 7 times larger. This is thought to be due to average flight distance being context-dependent, influenced by factors such as local resource availability (Zurbuchen et al. 2010). If there are appropriate resources nearby to the nest, then females will reduce their energy expenditure and fitness costs by not travelling to flower patches further away (Zurbuchen et al. 2010).
In this study A. retusa were found to have lower average flight distances than their estimated maximum, with an average distance of 122 m. This agrees with the results found in Hofmann et al. (2020). For example, Osmia cornuta, a slightly smaller species than A. retusa, was found to have an average foraging distance of 107 m with the maximum distance observed being 724 m. The shorter flight distances seen by A. retusa may be due to the species being polylectic and that there was abundant forage found throughout the 30 ha where they were observed in this study.
As the distances in this study were calculated between site of initial capture and subsequent recaptures, low distances may also be due to individuals showing site fidelity. Honey bees and bumblebee species are known to show site fidelity to a rewarding food source (Heinrich 1976; Comba 1999) where individuals will consistently travel to a patch of flowers even once they have finished flowering. A. retusa individuals were often caught either on the same patch of flowers or within a few meters as on previous survey days, perhaps indicating a similar behaviour.
Additional surveys outside of the core 25 ha identified female A. retusa foraging on either large patches of G. hedereceae or E. vulgare within 250 m of the original survey area. Beyond this point none were seen, perhaps indicating they are restricted to a specific area of the reserve (approximately 30 ha). A mark recapture study on the rare solitary bee Andrena hattorfiana examined small location populations and the movement of individuals between them. Most populations contained fewer than 50 females, with the average distance travelled between plant patches often less than 50 m (Franzén et al. 2009), a similar finding to the distances seen by foraging A. retusa, with most individuals caught less than 30 m from their original capture locations. On the western side of the reserve the habitat is predominantly scrub, with the Seaford Golf Course located throughout. The scrub is similar in floral composition to that found in the core study area, with patches of G. hedereceae present. However, the beginning of the scrub habitat where floral resources are most abundant is over 1 km from the potential nesting site, with a large section of arable land between these points. In Franzén et al. (2009) female A. hattorfiana rarely crossed areas where their pollen plant was not located, even if the distance was only 10 m. The lack of captures of A. retusa in the areas surrounding the reserve indicate that perhaps the population at Seaford head is isolated and restricted due to females not wanting to cross patches of land with minimal or no forage available.
Since A. retusa forage on common wildflowers, why is the population at Seaford Head small, and why is this one of the few sites where it occurs in Britain? No definitive answer can be given at this stage. Not only are floral resources essential for solitary bees but also appropriate nesting habitat (Franzén et al. 2009). The population at Seaford head are thought to be nesting in loess, the sandy deposits exposed at the top of the cliff face (Edwards and Jenner 2008; Steven Falk 2019 pers comms.) with two mating pairs found along the cliff edge in 2019. There is little information on the historical populations of A. retusa, therefore understanding why they have been lost from other sites in Britain is not possible. The sites where they remain and where information is available appear to be mainly coastal and are either protected or managed by The National Trust or through nature reserve status (Culver Down & Seaford Head) or in an area with little human intervention that harms wildlife (Lulworth Ranges, MOD owned, Dorset Hanson quarry site). The coastal populations have access to chalk cliffs, like those at Seaford head, with the population at the Hanson quarry site having access to soft, sandy, disturbed banks. Other locations the species is thought to be currently found, and where it has been identified in the past, include heathland. Here the soil, although not formed from loess, shares similar characteristics such as being free-draining and sandy (Pywell et al. 1994). More studies on these populations and locating the specific nesting aggregations is needed to help understand the nesting requirements and how this may be contributing to the species decline. Having preference for nesting sites is seen in other solitary bee species. Criteria include moist, compact soil with little or no vegetation (Wuellner 1999) or in other species soft soil on south facing slopes (Potts and Wilmer 1997). These contrasting criteria in different species highlights the importance of understanding the species-specific nesting requirements.
Although there is still more information required on A. retusa, our data highlights the importance of conserving coastal habitats and the abundant wild flower species often found there. For A. retusa appropriate forage available in early spring, particularly. G. hederacea, and later in the species flight season the presence of I. foetidissima and Fabaceae appears to be important for the Seaford Head population.
Conservation success stories, such as for the Large Blue butterfly, with its successful reintroduction (Thomas 1995), give hope for rare species like A. retusa and emphasize the importance and value of studying local populations and the specific needs of different species. With it only found in a few, isolated populations, it is at great risk of extinction in Britain. This study has highlighted that more research is required on this species to fully understand the cause of its rapid decline and that the conservation of the sites where it is currently located is vital for the species survival in Britain.
References
Aubert M (2013) Anthophora retusa. https://www.bwars.com/bee/apidae/anthophora-plumipes. Accessed 12 Dec 2019
BBC. Second search at Seaford Head after further cliff fall (2017) https://www.bbc.co.uk/news/uk-england-sussex-40371106. Accessed 19 Dec 2019
Beebee TJ, Griffiths RA (2005) The amphibian decline crisis: a watershed for conservation biology? Biol Conserv 125:271–285
Biesmeijer JC, Roberts SP, Reemer M, Ohlemüller R, Edwards M, Peeters T, Settele J (2006) Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313:351–354
Biological Records Centre. Glechoma hederacea. https://www.brc.ac.uk/plantatlas/plant/glechoma-hederacea. Accessed 15 June 2020
Brown A, Gilbert G, Wotton S (2012) Bitterns and bittern conservation in the UK. Br Birds 105:58–87
Buchmann S, Ascher JS (2005) The plight of pollinating bees. Bee World 86:71–74. https://doi.org/10.1080/0005772X.2005.11417316
Butterfly Conservation Trust. Duke of Burgundy. https://butterfly-conservation.org/butterflies/duke-of-burgundy. Accessed 8 Jan 2020
Cameron SA, Lozier JD, Strange JP, Koch JB, Cordes N, Solter LF, Griswold TL (2011) Patterns of widespread decline in North American bumble bees. Proc Natl Acad Sci 108:662–667
Comba L (1999) Patch use by bumblebees (Hymenoptera Apidae): temperature, wind, flower density and traplining. Ethol Ecol Evol 11:243–264
Croxton PJ, Hann JP, Greatorex-Davies JN, Sparks TH (2005) Linear hotspots? The floral and butterfly diversity of green lanes. Biol Conserv 121:579–584
de Oliveira Francisco F, Arias MC (2010) Inferences of evolutionary and ecological events that influenced the population structure of Plebeia remota, a stingless bee from Brazil. Apidologie 41:216–224
Dunn RR (2005) Modern insect extinctions, the neglected majority. Conserv Biol 19:1030–1036
Edwards M, Jenner M (2008) Anthophora retusa: the Potter Flower Bee. Hymettus. http://hymettus.org.uk/downloads/A%20retusa%202008.pdf. Accessed 19 Aug 2020
Eickwort GC, Ginsberg HS (1980) Foraging and mating behaviour in Apoidea. Annu Rev Entomol 25:421–446
Ellis S, Wainwright D, Dennis EB, Bourn NAD, Bulman CR, Hobson R, Jones R, Middlebrook I, Plackett J, Smith RG, Wain M (2019) Are habitat changes driving the decline of the UK’s most threatened butterfly: the High Brown Fritillary Argynnis adippe (Lepidoptera: Nymphalidae)? J Insect Conserv 23:351–367
Evans RL, Potts SG (2004) Iconic bees: South East England. Potter Flower Bee. Friends of the Earth
Falk S (1991) A review of the scarce and threatened bees, wasps and ants of Great Britain (No. 35). Nature Conservancy Council for England
Falk S, Lewington R (2015) Field guide to the bees of great britain and ireland. BLOOMSBURY WILDLIFE, London
Fisher RA (1930) The genetical theory of natural selection. Clarendon, Oxford
Franzén M, Larsson M, Nilsson SG (2009) Small local population sizes and high habitat patch fidelity in a specialised solitary bee. J Insect Conserv 13:89–95
Frechen M (2011) Loess in Europe. Quat Sci J 60:3–5
Gathmann A, Tscharntke T (2002) Foraging ranges of solitary bees. J Anim Ecol 71:757–764
Goulson D (1999) Foraging strategies of insects for gathering nectar and pollen, and implications for plant ecology and evolution. Perspect Plant Ecol 2:185–209
Goulson D, Lye GC, Darvill B (2008) Decline and conservation of bumble bees. Annu Rev Entomol 53:191–208
Goulson D, Nicholls E, Botías C, Rotheray EL (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science. https://doi.org/10.1126/science.1255957
Greenleaf SS, Kremen C (2006) Wild bee species increase tomato production and respond differently to surrounding land use in Northern California. Biol Conserv 133:81–87
Greenleaf SS, Williams NM, Winfree R, Kremen C (2007) Bee foraging ranges and their relationship to body size. Oecologia 153:589–596
Hampshire biodiversity action plant (2000) https://documents.hants.gov.uk/biodiversity/HampshireBiodiversityActionPlanVolume2.pdf. Accessed 29 Nov 2020
Hegland SJ, Boeke L (2006) Relationships between the density and diversity of floral resources and flower visitor activity in a temperate grassland community. Ecol Entomol 31:532–538
Heinrich B (1976) The foraging specializations of individual bumblebees. Ecol Monogr 46:105–128
Hofmann MM, Fleischmann A, Renner SS (2020) Foraging distances in six species of solitary bees with body lengths of 6 to 15 mm, inferred from individual tagging, suggest 150 m-rule-of-thumb for flower strip distances. J Hymenopt Res 77:105
Holzschuh A, Dudenhöffer JH, Tscharntke T (2012) Landscapes with wild bee habitats enhance pollination, fruit set and yield of sweet cherry. Biol Conserv 153:101–107
Horsley C, Whitehouse A, Falk S (2013) South West Bees Project
Hurst MD, Rood DH, Ellis MA, Anderson RS, Dornbusch U (2016) Recent acceleration in coastal cliff retreat rates on the south coast of Great Britain. Proc Natl Acad Sci 113:13336–13341
Jackson L (2019) East of England Bee Report: a report on the status of threatened bees in the region with recommendations for conservation action. Buglife - The Invertebrate Conservation Trust, Peterborough
Jones N (2013) Anthophora plumipies female. https://www.bwars.com/bee/apidae/anthophora-plumipes. Accessed 12 Dec 2019
Klein AM, Steffan–Dewenter I, Tscharntke T (2003) Fruit set of highland coffee increases with the diversity of pollinating bees. Proc R Soc Lond B 270:955–961
Koeniger N, Koeniger G (2000) Reproductive isolation among species of the genus Apis. Apidologie 31:313–339
Lande R (1993) Risks of population extinction from demographic and environmental stochasticity and random catastrophes. Am Nat 142:911–927
McKnight DT, Ligon DB (2017) Correcting for unequal catchability in sex ratio and population size estimates. PloS ONE 12:e0184101
McNeilage A, Robbins MM, Gray M, Olupot W, Babaasa D, Bitariho R, … Baker J (2006) Census of the mountain gorilla Gorilla beringei beringei population in Bwindi Impenetrable National Park, Uganda. Oryx 40:419–427
Meeus I, Brown MJ, De Graaf DC, Smagghe GUY (2011) Effects of invasive parasites on bumble bee declines. Conserv Biol 25:662–671
Mueller A, Kuhlmann M (2008) Pollen hosts of western palaearctic bees of the genus Colletes (Hymenoptera: Colletidae): the Asteraceae paradox. Biol J Linn Soc 95:719–733
National Research Council (2007) Status of Pollinators in North America. The National Academies Press, Washington, DC. https://doi.org/10.17226/11761
Natural England (2011) Habitat of the month: June 2011 Lowland meadows. http://webarchive.nationalarchives. gov.uk/20130903135107/http://www. naturalengland.org.uk/ourwork/conservation/biodiversity/englands/habitatofthemonth/lowlandmeadows.aspx. Accessed 1 Jan 2020
Nieto A, Roberts SPM, Kemp J, Rasmont P, Kuhlmann M, García Criado M, Biesmeijer JC, Bogusch P, Dathe HH, De la Rúa P, De Meulemeester T, Dehon M, Dewulf A, Ortiz-Sánchez FJ, Lhomme P, Pauly A, Potts SG, Praz C, Quaranta M, Radchenko VG, Scheuchl E, Smit J, Straka J, Terzo M, Tomozii B, Window J, Michez D (2014) European Red List of bees. Publication Office of the European Union, Luxembourg
Ollerton J, Winfree R, Tarrant S (2011) How many flowering plants are pollinated by animals? Oikos 120:321–326
Ollerton J, Erenler H, Edwards M, Crockett R (2014) Extinctions of aculeate pollinators in Britain and the role of large-scale agricultural changes. Science 346:1360–1362
Owens N (2011) Male Anthophora plumipies. https://www.bwars.com/bee/apidae/anthophora-plumipes. Accessed 12 Dec 2019
Peakall R, Schiestl FP (2004) A mark-recapture study of male Colletes cunicularius bees: implications for pollination by sexual deception. Behav Ecol Sociobiol 56:579–584
Potts S, Willmer PAT (1997) Abiotic and biotic factors influencing nest-site selection by Halictus rubicundus, a ground‐nesting halictine bee. Ecol Entomol 22:319–328
Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25:345–353. https://doi.org/10.1016/j.tree.2010.01.007
Powney GD, Carvell C, Edwards M, Morris RK, Roy HE, Woodcock BA, Isaac NJ (2019) Widespread losses of pollinating insects in Britain. Nat Commun 10:1018. https://doi.org/10.1038/s41467-019-08974-9
Purvis A, Gittleman JL, Cowlishaw G, Mace GM (2000) Predicting extinction risk in declining species. Proc R Soc Lond B 267:1947–1952
Pywell RF, Webb NR, Putwain PD (1994) Soil fertility and its implications for the restoration of heathland on farmland in southern Britain. Biol Conserv 70:169–181
Ricketts TH, Daily GC, Ehrlich PR, Michener CD (2004) Economic value of tropical forest to coffee production. Proc Natl Acad Sci 101:12579–12582
Rollin O, Bretagnolle V, Decourtye A, Aptel J, Michel N, Vaissière BE, Henry M (2013) Differences of floral resource use between honey bees and wild bees in an intensive farming system. Agric Ecosyst Environ 179:78–86
Schultz CB, Dlugosch KM (1999) Nectar and hostplant scarcity limit populations of an endangered Oregon butterfly. Oecologia 119:231–238
Schwarz CJ, Arnason AN (1996) A general methodology for the analysis of capture-recapture experiments in open populations. Biometrics 52:860–873
Shaffer ML (1981) Minimum population sizes for species conservation. Bioscience 31:131–134
Singh HS, Gibson L (2011) A conservation success story in the otherwise dire megafauna extinction crisis: the Asiatic lion (Panthera leo persica) of Gir forest. Biol Conserv 144:1753–1757
Sussex Wildlife trust. Seaford Head Nature Reserve. https://assets.sussexwildlifetrust.org.uk/swtseafordheadwalkleafletwebonly.pdf . Accessed 8 Jan 2020
Sussex Wildlife Trust Information about the Seven Sisters and Seaford Head https://assets.sussexwildlifetrust.org.uk/Files/information-about-the-seven-sisters-and-seaford-head.pdf. Accessed 19 Dec 2019
The Guardian (2015) Stinging indictment: poll reveals public ignorance about bees in the UK. https://www.theguardian.com/environment/2015/aug/18/stinging-indictment-poll-reveals-public-ignorance-about-bees-in-the-uk. Accessed 16 Dec 2019
Thomas JA (1995) The ecology and conservation of Maculinea arion and other European species of large blue butterfly. In: Ecol. conser. of butterflies, pp 180–197
Thomas CD (2000) Dispersal and extinction in fragmented landscapes. Proc R Soc 267(1439):139–145
Thomas JA, Telfer MG, Roy DB, Preston CD, Greenwood JJD, Asher J, Lawton JH (2004) Comparative losses of British butterflies, birds, and plants and the global extinction crisis. Science 303:1879–1881
Traill LW, Bradshaw CJ, Brook BW (2007) Minimum viable population size: a meta-analysis of 30 years of published estimates. Biol Conserv 139:159–166
Wcislo WT, Cane JH (1996) Floral resource utilization by solitary bees (Hymenoptera: Apoidea) and exploitation of their stored foods by natural enemies. Annu Rev Entomol 41:257–286
Westrich P (1996) Habitat requirements of central European bees and the problems of partial habitats. Linnean Soc 18:1–16
Winfree R, Aguilar R, Vázquez DP, LeBuhn G, Aizen MA (2009) A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology 90:2068–2076. https://doi.org/10.1890/08-1245.1
Woodcock BA, Bullock JM, Shore RF, Heard MS, Pereira MG, Redhead J et al (2017) Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science 356:1393–1395
Wood TJ, Holland JM, Goulson D (2017) Providing foraging resources for solitary bees on farmland: current schemes for pollinators benefit a limited suite of species. J Appl Ecol 54:323–333
Wood TJ, Kaplan I, Szendrei Z (2018) Wild bee pollen diets reveal patterns of seasonal foraging resources for honey bees. Front Ecol Evol 6:210
Wuellner CT (1999) Nest site preference and success in a gregarious, ground-nesting bee Dieunomia triangulifera. Ecol Entomol 24:471–479
Zayed A, Packer L (2007) The population genetics of a solitary oligolectic sweat bee, Lasioglossum (Sphecodogastra) oenotherae (Hymenoptera: Halictidae). Heredity 99:397
Zurbuchen A, Landert L, Klaiber J, Müller A, Hein S, Dorn S (2010) Maximum foraging ranges in solitary bees: only few individuals have the capability to cover long foraging distances. Biol Conserv 143:669–676
Acknowledgements
The authors would like to thank Sussex Wildlife trust for their permission to work within the Seaford Head reserve, with particular thanks to Graeme Lyons for his additional survey work and assistance in finding and identifying A. retusa. We would like to give special thanks to Mike Edwards from BWARS and Steven Falk their detailed and helpful information on A. retusa populations throughout Britain. Thank you to Hanson Aggregates in Dorset and Andy Duncan for allowing us to visit the quarry site. Thank you to our funders, Gatwick Airport and the University of Sussex Doctoral School. Thank you to the reviewers for their insightful and helpful comments. Finally, a thank you to those who assisted with data collection and providing advice; Jay Pugsley, Kyle Shackleton, Veronica Wignall and Tobias Collingwood.
Funding
GH was funded by Gatwick Airport and the University of Sussex Doctoral School.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Due to the study system being invertebrates and no destructive sampling was used ethical approval was not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hennessy, G., Goulson, D. & Ratnieks, F.L.W. Population assessment and foraging ecology of the rare solitary bee Anthophora retusa at Seaford Head Nature reserve. J Insect Conserv 25, 49–63 (2021). https://doi.org/10.1007/s10841-020-00272-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-020-00272-w