Abstract
Many Bumble bee (Bombus) species are in decline and conservation efforts must be undertaken now to lessen or reverse the trend. For effective efforts to occur, the first step must be an accurate assessment of extinction risk. Yet only four of over forty Canadian Bombus species have been assessed by the Committee on the Status of Endangered Wildlife in Canada (COSEWIC), despite evidence of decline for numerous species in this genus. Here, we evaluated the status of the American Bumble bee, Bombus pensylvanicus De Geer 1773 in Canada. A challenge with species assessments is obtaining adequate occurrence data temporally and spatially. Citizen science is a field where volunteers can collect data similar to that of experts over a broader coverage than researchers could often cover alone. We used data from the Bumble Bee Watch citizen science program, a database of North American Bombus records representing field survey and collection records from the late-1800s, and our own field surveys to evaluate the status of B. pensylvanicus in Canada using the International Union for the Conservation of Nature (IUCN) Red List assessment criteria. We found that B. pensylvanicus’ Area of Occurrence has decreased by about 70%, its Extent of Occurrence by 37%, and its relative abundance by 89%, from 2007 to 2016 as compared to 1907–2006. These findings warrant an assessment of Critically Endangered using IUCN Red List criteria for B. pensylvanicus in Canada. Our findings will help inform management of B. pensylvanicus and exemplify the importance of citizen science programs for wildlife conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Effective conservation management of at-risk species requires the important first step of an accurate assessment of extinction risk (Rodrigues et al. 2006; Mace et al. 2008; Cardoso et al. 2011; Colla 2016). Currently in Canada, there are several federal and provincial policies considering pollinator conservation management (Parliament of Canada 2015; Government of Ontario 2016; Agriculture and Agri-Food Canada 2017; Health Canada 2017). This is a considerable improvement since an earlier review found no real protection for pollinators in the legislation (Tang et al. 2007). In this paper, we focus on how, despite evidence of decline at various scales throughout its range and assessment of Vulnerable by the IUCN Red List (Hatfield et al. 2015c), an assessment of extinction risk in Canada has not yet been completed and made available for government and public consideration for the American Bumble bee, Bombus pensylvanicus De Geer 1773.
Bombus pensylvanicus is widespread in eastern, central, and western US, and can be found in several Mexican states (Williams et al. 2014). In Canada, it is known from southern Ontario and Quebec (Williams et al. 2014); a previous record of it from Alberta as shown in Williams et al. (2014) was determined to be a misidentification (personal communication, C. Sheffield, Curator of Invertebrate Zoology, Royal Saskatchewan Museum). Bombus p. sonorus is found in the southwestern United States and has a lighter colour pattern than B. pensylvanicus in the east; the two groups appear to separate out in preliminary DNA barcoding analyses, but as there are potential intermediary groups not yet barcoded, we follow the treatment of Williams et al. (2014) by including B. p. sonorus as a subspecies of B. pensylvanicus (Williams et al. 2014).
Bombus pensylvanicus is a grassland species associated with open fields and farmlands (Colla and Dumesh 2010; Williams et al. 2014). In its Canadian range, it is a late-emerging species that tends to nest on the surface of the ground amidst long grasses, occasionally nests above ground, and only rarely nests underground (Wyatt 1970; Macfarlane 1974; Macfarlane et al. 1994; Colla and Dumesh 2010; Williams et al. 2014). This species tends to have smaller colonies (average of 33 new queens and 132 workers and males produced per colony) relative to congeners (Macfarlane et al. 1994). It is also more aggressive than those Bombus species that nest underground (Macfarlane et al. 1994), as has been seen in some but not all other surface-nesting Bombus (Macfarlane et al. 1994; Schweitzer et al. 2012) potentially because of the increased predation risk these nests face. The aggression may also depend on the sub-genera and location, with researchers indicating species such as B. pensylvanicus and others in the Thoracobombus subgenus being aggressive in North America but not in Britain (Cameron et al. 2001). In Ontario, queens first appear in mid to late May, with workers appearing within a month; males emerge in August and new queens emerge from late August to September, with the colonies wrapping up by the end of September (Wyatt 1970; Macfarlane et al. 1994).
The Mixed-wood Plains Ecozone is contained primarily (73%) in the southern portion of Ontario, but also extends into the extreme southern portion of Quebec (ESTR Secretariat 2016). It represents approximately 8% of Ontario’s landmass yet contains about 92% of its human population, which is reflected in the dense coverage (68%) of anthropogenic land uses types, particularly agricultural, in that Ecozone (Ontario Biodiversity Council 2010, 2011; Statistics Canada 2016). While it is amongst the most species-rich regions of Canada, 73% of all Ontario species of conservation concern and 75% of its rare ecosystems are located in this Ecozone (Ontario Biodiversity Council 2010). Threats to species in this area include habitat loss, invasive alien species, human population growth, pollution, unsustainable land use, and climate change (Ontario Biodiversity Council 2010, 2011; ESTR Secretariat 2016).
Although Bumble bees are known to live in patchy habitats (e.g. agricultural areas, urban areas), isolated or poor quality habitats may not be able to support Bumble bee populations (Hatfield and LeBuhn 2007; Iles et al. 2018). Additional threats to Bumble bees like B. pensylvanicus may include parasites and pathogens, such as the fungal pathogen Nosema bombi, including those spread from managed commercial Bumble bee and honeybee colonies (Colla et al. 2006; Hoffmann et al. 2008; Otterstatter and Thomson 2008; Gillespie 2010; Cameron et al. 2011a, 2016; Szabo et al. 2012; Graystock et al. 2016). Pesticides have been and still are a threat to bees, from past use of organophosphorus and carbamate compounds (Kevan 1975; Kevan et al. 1984, 1997) to the current widespread use of systemic neonicotinoid insecticides and fungicides; neonicotinoids have been used increasingly since the 1990 s with mounting evidence showing negative impacts on Bumble bees, particularly sub-lethal effects (Gill et al. 2012; Szabo et al. 2012; Whitehorn et al. 2012; Godfray et al. 2014, 2015; Goulson 2015; Lundin et al. 2015; Wood and Goulson 2017).
Conservation assessments require occurrence data from all parts of a species’ range, which can be a challenge for species with a large distribution. These assessments also require data coverage across time. Citizen science is a growing field that may help to increase data collection as volunteers can collect data similar or identical to that of experts, particularly as programs become more accessible through the use of the Internet and technology (websites, photos, smartphone apps, etc.) (Silvertown 2009; Conrad and Hilchey 2011; Follett and Strezov 2015). The success of the program and quality of data does depend on the complexity of information being collected and skills needed (Kremen et al. 2011).
Although most insect taxa require physical specimens to be collected in order for identifications to be made (Kremen et al. 2011), Bumble bees can sometimes be identified to species by photos (Lye et al. 2012; Richardson et al. 2019; van der Wal et al. 2015; Beckham and Atkinson 2017; The Xerces Society for Invertebrate Conservation, Wildlife Preservation Canada, York University, et al. 2017). For example, 75% of the Bumble bee observations submitted to the iNaturalist Vermont site were identified to species through photos (Mcfarland et al. 2016), and, as of January 31, 2018, 86% of all Bumble bee observations that had been submitted to Bumble Bee Watch and reviewed by an expert were identified to species (those records that were assigned a tentative identification were considered to not be identified to species, and both pending and invalid submissions (those that were not actually Bumble bees) were excluded from this analysis) (The Xerces Society for Invertebrate Conservation, Wildlife Preservation Canada, York University, et al. 2018).
Previous work in the UK with the BeeWatch program (Lye et al. 2012; van der Wal et al. 2015), the Texas Bumblebees Facebook page, and the iNaturalist Bees and Wasps of Texas project (Beckham and Atkinson 2017) suggests that citizen science projects based on photo submissions of bees can capture data complementary to that collected by experts and assist in learning more about, and tracking changes to, species over time. Bumble Bee Watch is a web-based citizen science program where participants photograph Bumble bees anywhere in North America, upload the photos and relevant site information to a website or through a hand held device, and work through an interactive identification key to arrive at a species name, which experts then verify (The Xerces Society for Invertebrate Conservation, Wildlife Preservation Canada, York University, et al. 2017). We used this data set in conjunction with expert-collected data to aid in the assessment of conservation status for B. pensylvanicus in Canada using the IUCN Red List criteria.
Methods
Data compilation
We obtained data from three sources for Ontario and Quebec, dating to the end of 2016: 1) The Bumble bees of North America (BBNA) database; 2) Records from the Bumble Bee Watch citizen science program already verified by experts, particularly 2014–2016; and 3) Field surveys conducted by the authors dating to the early 2000s, particularly 2013–2016, excluding those records already incorporated into the BBNA database.
Bumble bees of North America (BBNA) database
LLR maintains a database of North American Bumble bee species records assembled from numerous private, research, and other collections (see http://www.leifrichardson.org/bbna.html). This dataset was originally assembled to support the development and publication of the Bumble bees of North America (Williams et al. 2014), with new records added each year to support a variety of species status assessments (e.g. IUCN North American Red List Bombus Assessment (Hatfield et al. 2015b, 2015c, 2015d) and the United States Fish and Wildlife Service (USFWS) assessments (Defenders of Wildlife 2015; Szymanski et al. 2016)). An export was obtained from the database on May 12, 2016 for Ontario (18,986 records) and May 23, 2017 for Quebec (4470 records); no further records for Ontario had been added between May 12, 2016 and May 23, 2017 so a newer export was not obtained.
We excluded duplicate occurrences found in the data set, as well as those found in other data sets used in the project, including some of SRC’s research collections and Bumble Bee Watch data. Records with missing or incorrect geographic coordinates as determined by a visual review of the data were updated based on label data or removed. We further updated three records erroneously identified (as determined by SRC) as B. pensylvanicus from Grassy (Reed) Narrows (two records) and Thessalon (near Sault St. Marie) (one record) to be Bombus sp.
Bumble Bee watch data
All records submitted to the Bumble Bee Watch citizen science program that had been verified by experts to date were exported on May 12, 2017. This included 78 records from Quebec and 2092 records for Ontario (2170 total). Data were screened and corrected as described per the BBNA database.
MacPhail and colla field surveys
Bumble bee surveys by the authors and colleagues were conducted at sites in southern and central Ontario and the extreme south of Quebec over the last decade (see Table ESM.1 and Fig. ESM.1 in the Online Resource). These included targeted surveys for known species at risk of extinction, such as at historic and extant B. affinis Cresson sites in 2007–2016, and known and potential B. terricola Kirby sites in 2014–2016, and B. bohemicus Seidl (= B. ashtoni Cresson) sites in 2016. Surveys generally took place between 7 am and 7 pm on precipitation-free days between 3 and 30 °C from April to October, with the majority of surveys occurring during July and August. Bees were usually collected using sweep nets, identified, and released at the end of the survey period, although some were identified on the wing. Photos were also taken of some specimens for later review. Identification was based on keys in Laverty and Harder (1988) and Williams et al. (2014), as well as comparison to physical specimens in reference collections held at the University of Guelph and York University. Bumble bee data were also collected from pollinator surveys completed in 2010–2011 in Algonquin Provincial Park (Nardone 2013). Data collected by the authors previously incorporated into the BBNA database are not included in this source category to avoid repeating the same datum.
Data summary
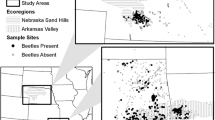
After data compilation and cleaning, there were 34,221 Bombus records from 1907 to 2016 over all three data sets (Table 1). While the majority of Bombus records were from southern Ontario, southern Quebec and central Ontario also had fair representation, while the northern regions had few observations (Fig. 1). Despite the extensive data set and survey effort, B. pensylvanicus was only found from southern Ontario and extreme south-western Quebec (Fig. 1).
All Bombus spp. (open symbols) and B. pensylvanicus (closed symbols) observations in a historic (1907–2006) and b recent (2007–2016) period in Ontario and Quebec, based on the three data sources as described in the text (BBNA database, Bumble Bee Watch citizen science program, MacPhail and Colla surveys)
Data were limited and categorized into historic and recent time periods (Table 1). The 1907–2006 period was chosen to provide a one hundred year historic period (152 total Bombus records pre-1907 were excluded from the BBNA dataset, including 4 B. pensylvanicus), which provides an extensive baseline for comparison to modern times. The 2007–2016 period meets the IUCN red list criterion A2 (population reduction) that required declines to have been observed over the last 10 years (IUCN 2012a; IUCN Standards and Petitions Subcommittee 2016).
Records that did not have a year associated with the observation but were known to occur pre-2007 (based on data associated with the record, such as the collector name or date the identification was determined) were included in the timeframe for analyses where possible. For example, these data were included for calculations of Extent of Occurrence and changes in relative abundance between the two main periods (as they were known to have occurred pre-2007), but not for calculations of relative abundances per decade (as they could not be assigned to a specific decade).
Limiting of all Bombus spp. data to B. pensylvanicus range extent
We used ArcGIS 10.5 (ESRI 2016) to build a minimum convex polygon around the 1907–2016 Canadian observations of B. pensylvanicus (see Table ESM.2 for a breakdown of records per data source), then clipped the full 1907–2016 Bombus dataset to this polygon to create a subset of the Ontario and Quebec Bombus observations (Fig. 2, Table 1). The resulting subset of data included 7579 historic records and 11,895 recent records (Table 2), with the number of observations and relative abundance varying by decade (Table 3).
Analysis of data
IUCN criteria
We use the globally recognized, standardized, and comprehensive IUCN Red List Criteria (Rodrigues et al. 2006; Mace et al. 2008; IUCN 2012a; IUCN Standards and Petitions Subcommittee 2016) specifically their regional guidelines (IUCN 2012b) to calculate the conservation status or risk of extinction for B. pensylvanicus. As per the assessment process described in IUCN (2012b) and IUCN Standards and Petitions Subcommitee (2016), each criterion (e.g. population reduction, small geographic area) was evaluated if possible, and the highest threat category (e.g. Endangered vs Vulnerable) from all of these evaluations was assigned to the species. We focused on IUCN Criterion A2, which relates to population reduction (measured over the last 10 years) observed, estimated, inferred, or suspected in the past where the causes of reduction may not have ceased or may not be understood or may not be reversible. A2b is an index of abundance appropriate to the taxon, for which we have used relative abundance relative to historic values to evaluate. A2c relates to a decline in Area of Occurrence, Extent of Occurrence, or habitat quality: we use both Area of Occurrence and Extent of Occurrence here. For each A2 category, population reduction must be ≥ 80% to be critically endangered, ≥ 50% to be endangered, or ≥ 30% to be vulnerable.
To calculate changes in spatial distribution, we calculated the Area of Occurrence (AOO) and Extent of Occurrence (EOO) for each of the two B. pensylvanicus data sets as previously described (historic 1907–2006 and recent 2007–2016 periods). The EOO is measured as the area within the smallest boundary that can be drawn around the edge of all known or expected occurrences, while the AOO is a measurement of the space a species actually occupies, based on the number of 2 km wide grid cells that it occupies within the EOO (IUCN 2012a). Each data set was imported into the online GeoCAT geospatial conservation assessment tool (Bachman et al. 2011), which provided calculated values for AOO and EOO based on a minimum convex hull polygon. The GeoCat results do include portions of the Great Lakes, which are not habitat for Bumble bees. The inclusion of these areas of unsuitable habitat in the calculations for EOO is encouraged by some authors (Gaston and Fuller 2009; Bachman et al. 2011) and in the GeoCAT program itself (Royal Botanic Gardens Kew 2017), which reference the discussion about the difference in EOO versus AOO (the AOO does not include any grid cells the species does not occur in so by default does not include areas of unsuitable habitat). While the IUCN indicates that either method is acceptable (IUCN 2012a), other authors, such as Hatfield et al. (2015a) indicate that these areas should be removed.
We therefore also calculated Extent of Occurrence in ArcGIS (ESRI 2016) using the minimum bounding geometry tool (with convex hull option) to calculate the area with and without the Great Lakes, to determine if the inclusion of these discontinuities or disjunctions affected the final status assessment. We found that the two ArcGIS approaches resulted in a similar final change in EOO and was also similar to the GeoCat approach (see Table ESM.3). Thus, we will present results following the GeoCat approach in this paper.
Relative abundance can be used to compare data sets with unequal sample sizes, and to evaluate changes over time (Colla et al. 2012; Hatfield et al. 2015a; Jacobson et al. 2018; Richardson et al. 2019). To calculate changes in the relative abundance of B. pensylvanicus before and after 2007, the total number of B. pensylvanicus observations was divided by the total number of all Bombus observed in that period (1907–2006 and 2007–2016, and each individual decade). Linear regression was used to infer the rate of change in relative abundance over time. Two-tailed z-tests of equal proportions were used to determine if the relative abundance differed significantly between the overall historic and recent periods, and between adjacent decades. Microsoft Excel (Microsoft Office Professional Plus 2013) was used to calculate the relative abundances for each period, to plot the linear regression equation as calculated using SPSS (IBM SPSS Statistics ver. 24), and to plot other graphs in this document. SPSS was also used to calculate the z test values.
To determine if immigration of B. pensylvanicus from outside of its Canadian range could occur and help lower its extinction risk (“rescue effect”(IUCN 2012b)), the ability of the species to move across the landscape must be considered. While the dispersal distances of B. pensylvanicus specifically and Ontario Bombus generally are unknown, it could be up to 10 km per year based on that of other Bombus species (Kraus et al. 2009; Colla 2017). Rescue must therefore be from US states adjacent to Canada and not from other parts of B. pensylvanicus’ North American range. However, the rescue effect also depends on how the species is doing in the adjacent areas: if it is declining there, it will not likely to be able to help rescue the Ontario and Quebec populations. We evaluated the nearest states and their NatureServe (2015) ranking of status for this section. If it is found that a rescue effect could occur, then the Red List assessment should be downlisted by one category (e.g. from critically endangered to endangered) (IUCN 2012b).
Natural history information for B. pensylvanicus
In addition to analyses related to declines, we also compiled information about B. pensylvanicus’ biology and natural history from this data set. This included emergence periods of each caste, flight periods, and forage plants: important information for conservation efforts.
Results
Change in AOO, EOO
The Area of Occurrence for B. pensylvanicus declined by over 204 km2 or about 70% of its previous range (Table 4), and the Extent of Occurrence for B. pensylvanicus declined by over 38,000 km2 or about 37% of its previous range (Fig. 3, Table 4), between the historic 1907–2006 and recent 2007–2016 periods.
Relative abundance
A statistically significant decline of 89% in B. pensylvancus relative abundance within its Canadian range occurred between the historic (1907–2006) and recent (2007–2016) periods (Fig. 4, Table 5) (Z = 17.215; p < 0.0001). There was not a significant decline trend over the entire time period (R2 = 0.197, p = 0.172)(Fig. 5). However, there were increases and decreases in relative abundance per decade overtime (Fig. 5). Indeed, there were signficant differences in four pairs of decades: 1917 vs. 1927 (Z = 2.570, p = 0.001), 1927 vs. 1937 (Z = 4.026; p < 0.0001), 1947 vs. 1957 (Z = 2.462; p = 0.014), and 1977 vs. 1987 (Z = 3.378; p = 0.001) (see results for each set of decade comparisons in Table ESM.4).
A comparison of the mean relative percent abundance of B. pensylvanicus as compared to all Bombus spp. recorded in its range across Ontario and Quebec in historic (1907–2006) and recent (2007–2016) periods (n = 19,474). There were significantly fewer B. pensylvanicus relative to all Bombus spp. in the recent period as compared to the historic period (z-value 17.2145 p value < 0.0001). Different letters above the bars indicate significant differences
Rescue effort
The nearest states to the Canadian range of B. pensylvanicus, and their NatureServe (2015) subnational (S-rank) status rankings are: Maine (SH—possibly extirpated (historical)), Vermont (S1—critically imperiled), New York (S1—critically imperiled), Pennsylvania (SNR—not ranked), Ohio (no NatureServe listing), Michigan (SNR—not ranked). Bombus pensylvanicus is therefore considered to be in severe straits (extirpated or critically imperiled) in all of the adjacent states that have been ranked. It is possible that the unranked (SNR) populations are also at risk as a query of the BBNA database for the adjacent states shows only 13 recent observations (since 2006) of B. pensylvanicus (personal communication, L. Richardson, Gund Institute for Environment, University of Vermont). As well, a recent continent-level status assessment for NatureServe found that B. pensylvanicus has been uplisted to G3 (personal communication, L. Richardson, Gund Institute for Environment, University of Vermont) from G3G4 (NatureServe 2015). The overall rescue effect from the United States is therefore believed to be limited, and thus no adjustment to the calculated IUCN Red List regional assessment needs to be made (i.e. no uplisting or downlisting of categories).
Application of IUCN criteria
By applying the IUCN Red List Regional Guidelines to our assessment of this species, we find that it merits a status of critically endangered A2b due to a decline in relative abundance of over 80% (Table 6). Criterion A2c alone would merit a status of Vulnerable with a decline of over 30% in range size for EOO or Endangered for over 50% in range size for AOO (Table 6). While A2e also applies due to the suspected influence of introduced parasites and pathogens, including from commercial Bumble bee colonies and honeybee colonies (Colla et al. 2006; Otterstatter and Thomson 2008; Cameron et al. 2011b, 2016; Szabo et al. 2012; Graystock et al. 2016), this was not able to be quantified for the purposes of this study.
Other criteria were not applied as they were not relevant. For example, criterion B, C, and D were not relevant to B. pensylvanicus as the species has too great an EOO, population size, and number of locations, and E was not relevant as the criterion requires a 50% probability of extinction in the wild in the next 10 years, which is unlikely to happen based on current population size and trend.
Natural history information
The earliest spring record of B. pensylvanicus was May 15 and the latest record was October 5, with the most records reported in August (avg 1.1 bees per month per year ± 0.34 SE) (Fig. ESM.2). Unsurprisingly, workers were the most common caste recorded (56% of records), followed by queens and males (Table ESM.5). Queens begin to emerge in May and can be found through October (Fig. ESM.3). Workers emerge in June and males begin to emerge in July, with both still being found through October (Fig. ESM.3).
Very few records (9.2% or 32 of 329) actually had information about what plant the bee was collected off of so it is difficult to make any declarations about preferred host plants. The forage plant was only recorded for 31 observations and 1 record was noted as flying by. Although the most commonly visited species was Vicia cracca (cow vetch), this may be a site-specific finding as 19 of the 22 observations were from same the site. The other plant species noted included Centaurea maculosa (1 record), Cirsium vulgae (2 records), Monarda fistulosa (4 records), Stachys byzantini (1 record), and Trifolium pratense (1 record).
Discussion
Many Bumble bee species are currently in decline and conservation efforts must be undertaken now to lessen or reverse the trend. For effective efforts to take place, the first step must be an accurate assessment of extinction risk. Bombus pensylvanicus is just one species that has not yet been assessed by provincial or federal governments in Canada yet we found it to be Critically Endangered. A global risk assessment (Hatfield et al. 2015c) for B. pensylvanicus previously found it be Vulnerable, according to the IUCN Red List criterion A2be based on a > 30% reduction in population using relative abundance and persistence measurements, as well as a decrease in range and with the documented threat of pathogens. Hatfield et al. (2015c) had actually calculated a 51% reduction, which leads to an Endangered listing, but downlisted their assessment as they believed a Vulnerable listing was more representative of the true decline of the species. However, with B. pensylvanicus’ smaller range in Canada, the rapid declines seen in other Canadian Bumble bee species (e.g. B. affinis and B. bohemicus)(COSEWIC 2010, COSEWIC 2014a; Environment and Climate Change Canada 2016; Colla 2017), the knowledge that Bumble bee colonies at northern latitudes tend to have smaller colonies (Yalden 1982; Donovan and Wier 1984; Williams 1988; Macfarlane et al. 1994) and with climate change shown to be limiting rather than allowing a northward range expansion or elevational shift by Bumble bees (Kerr et al. 2015; Pyke et al. 2016), a more protective stance needs to be taken here in order to prevent extirpation.
Our study was the first to combine citizen science data with expert-collected and historic data to examine the status of a Bumble bee species across its Canadian range (citzen science data has been used for Bumble bees in Vermont (Richardson et al. 2019), Texas (Beckham and Atkinson 2017), and the UK (Lye et al. 2012). The data from Bumble Bee Watch was particularly valuable as it represented 20% of the recent (2007–2016) records of B. pensylvanicus (10/50 records) and 36% of the recent B. pensylvanicus locations (8/22 sites), even with the program not having been launched until 2014. The inclusion of these data led to a better understanding of its status, as the change in EOO between historic and recent times was found to be − 37.12% with the data as compared to − 53.65% without, and the decline in RA 88.6% with these data (and a 57% increase in sample size for all Bombus) as compared to an 89.2% decline in RA (and a 32% increase in sample size) without, again between historic and recent time periods. As the species is already at the northern edge of its North American range, its range to the west in Ontario is interrupted at Lake Huron, and the south is at the Canada–US border, the extent of occurrence for its Canadian range is not likely to change drastically. An additional benefit of this program will be an increase in information about B. pensylvanicus’ natural history, from activity times to forage plants being visited.
It is not yet clear why B. pensylvanicus is declining. It is suspected that different factors and interaction effects are involved: pesticides, habitat loss, climate change, parasites, diseases, non-native species, and low genetic diversity and effective population sizes may all play a role (Zayed and Packer 2005; Colla et al. 2006; Colla and Packer 2008; Hoffmann et al. 2008; Cameron et al. 2011b; Szabo et al. 2012; Burkle et al. 2013; Goulson et al. 2015; Hatfield et al. 2015c; Kerr et al. 2015; Colla and MacIvor 2016; Pyke et al. 2016). Indeed, these factors may effect species and populations differently or have synergistic or antagonistic effects. Additionally, Bumble bees with more specialized or narrower niches, as well as those at the edges of their ranges (thus including B. pensylvanicus), may be more vulnerable to decline than others (Williams et al. 2007; Arbetman et al. 2017), even without the stressor of climate change that is already causing Bumble bee species ranges to contract from the south, not expand to the north, and to not shift in elevation (Kerr et al. 2015; Pyke et al. 2016). While it is not clear if this is the reason for the decline in B. pensylvanicus (particularly considering the fact that it’s declining even in historically strong and central parts of its range), it does mean that conservation efforts must focus on supporting current populations and not anticipating immigration from the south or movement north.
Not all Bumble bee species are declining in Canada and globally, and there are trends to suggest that certain phylogenies may be more susceptible than others (Arbetman et al. 2017). Globally, the Thoracobombus subgenus of Bumble bees has been shown to have proportionally more species in decline than other subgenera (Arbetman et al. 2017). Bombus pensylvanicus is a member of the Thoracobombus subgenus, which also includes B. fervidus Fabricius (Williams et al. 2014), another grassland species shown to be Vulnerable across its range in North America (Hatfield et al. 2015b) but not yet assessed in Canada (Hatfield et al. 2015b). In Canada, the Bombus subgenus has been noted to be strongly in decline. Currently four Bumble bee species have been assessed by the Committee on the Status of Wildlife in Canada, and all have been found to be at risk of extinction. Three of these species, B. affinis, B. occidentalis Greene, and B. terricola, are all from the Bombus subgenus (Williams et al. 2014), while the fourth species, B. bohemicus (from the Psithyrus subgenus) is a social parasite of bees of that subgenus (COSEWIC 2010, 2014a, b, 2015). This suggests that there are differences in the evolutionary history of Bumble bees that are making some more vulnerable to stressors than others (Arbetman et al. 2017).
In Illinois, Lozier and Cameron (2009) found that B. pensylvanicus had lower genetic diversity than B. impatiens Cresson, as was found in later broader studies (Cameron et al. 2011b; Lozier et al. 2011). However, when a restriction site-associated DNA sequencing (RADseq) technique was used as compared to the previous microsatellite-based analyses, Lozier (2014) found little difference between these two species. Bees are known to experience rapid loss of diversity with a resulting increased risk of extinction with decreased population size and inbreeding due to the haplodiploid system of sex determination Hymenoptera have (Zayed and Packer 2005). Thus their populations can crash quickly, and action must be taken sooner rather than later to recover them.
Cameron et al. (2011b) and Gillespie (2010) found that declining species of Bumble bees, including B. pensylvanicus, had higher levels of the parasite Nosema bombi than stable species, although interestingly, Arbetman et al. (2017) found the opposite, that common species were more likely to carry parasite loads than declining ones. Gillespie (2010) found that there was no significant difference in conopid parasitism between rare species like B. pensylvanicus and common species, although there were significantly lower Crithidia parasitic infections in the rare species. It is possible that spill-over of Nosema from managed Bumble bee pollinators in greenhouses to wild populations (Colla et al. 2006; Otterstatter and Thomson 2008) is causing declines, as the decline in B. pensylvanicus was correlated with high densities of vegetable greenhouses (Szabo et al. 2012). Gillespie (2010) noted that sites over 3 km from any potential managed Bumble bee colony still showed high Crithidia and Nosema infection levels. A study in Southern Ontario and Quebec by Cheryomina (personal communication, M. Cheryomina (unpublished draft MSc thesis, York University, 2015) found no Nosema infection in six uncommon species, including B. pensylvanicus, while it was present in five common species; similarly, she found no tracheal mites in the uncommon species and only one individual of a declining species with conopid fly parasitism. It is possible that Cheryomina’s and Gillespie’s results were influenced by small sample size, with each study having less than ten individuals of each the uncommon species sampled (including B. pensylvanicus) as compared to the range of 5–379 (Cheryomina) and 7–557 (Gillespie) individuals of each species investigated overall. It is also possible that common species have more resistance or tolerance to these parasites, thus carrying them while still foraging, while declining species may not survive the infection and thus infected workers would not be found (Arbetman et al. 2017). Therefore the connection between infection and species decline is still uncertain.
As B. pensylvanicus is a grassland species, the extensive loss of grasslands in Ontario may be one reason why this species is in decline (Colla and Dumesh 2010; Ontario Biodiversity Council 2010, 2011; Hatfield et al. 2015c). A visual assessment using Google Maps and Google Streetview (Google 2018) of the habitat around the recent sites documented in this study found that 73% were located in, or within 500 m of, a grassland/old field area (including one pasture site), with an additional 18% of sites in, or within 200 m of, a large city park and/or a large riparian area. The remaining 10% of sites were either in an intense agricultural area (1 site) or urban area (1 site), with the latter just over 600 m from a riparian area. Bombus pensylvanicus was reported to use many anthropogenic sites (e.g. edge of agricultural fields, sheds, lofts) when it was more common (Macfarlane 1974; Macfarlane et al. 1994), which suggests the lack of natural sites currently may not be a limiting factor, but this study suggests that the current Canadian populations seem to prefer open un-mowed areas.
In addition to general habitat needs, such as for nesting, sufficient foraging resources are critical for Bumble bee species (Colla and Packer 2008; Potts et al. 2010; Burkle et al. 2013; Goulson et al. 2015; Colla 2016). Climate change can cause changes in phenology, or reduce the number of flowers or amount of flower provisions, in addition to effects of extreme weather events on plants and pollinators (Inouye 2008; Bartomeus et al. 2011; Kjohl et al. 2011; Goulson et al. 2015; Miller-Struttmann et al. 2015; Pyke et al. 2016). The loss of forage plants, particularly for specialist species, may result in a decline of the pollinator species (León-Cortés et al. 1999; Johnson and Steiner 2000; Memmott et al. 2007; Burkle et al. 2013). As a generalist species that has been known to feed on over 200 plant species (Macfarlane 1974; Colla and Dumesh 2010; Williams et al. 2014), B. pensylvanicus could be thought resilient to the loss of some plant species, as a result of disturbances such as habitat loss or climate change. However, although B. pensylvanicus was the bee species with the most observed unique plant species interactions in an Illinois study in the late 1800s, it was only observed once, on one plant species a century later at the same area, despite an extensive survey effort and with many of the same plants being in existence (Burkle et al. 2013). In our study, we only had information on plant forage for 31 B. pensylvanicus observations, but 71% of those were on Vicia cracca, a non-native species; interestingly, another recent study in southern Ontario found that at-risk Bombus, including B. pensylvancius, showed a preference for this species rather than native species in the same area as more common Bombus did, presenting an interesting conservation dilemma relating to planting of non-native species (Gibson et al. 2019). More information about its plant preferences and nest sites will be important to gather to aid in conservation efforts.
Neonicotinoid pesticides are a class of pesticides that have increasingly been flagged as an area of concern in relationship to pollinator health, including Bumble bees (although there is still debate as to their role in declines) (Gill et al. 2012; Szabo et al. 2012; Whitehorn et al. 2012; Godfray et al. 2014, 2015; Goulson 2015; Lundin et al. 2015; Wood and Goulson 2017). They have been used in Ontario since the 1990s in the agricultural industry in particular, with as much as 99% of all corn and 60% of all soybean seeds, representing about 4.9 million acres total, being treated with them annually (Government of Ontario 2014), although a regulation was introduced to try and limit the amount of crops treated starting in 2016 (Government of Ontario 2016). The area of the province these crops are grown in highest concentration includes the main region of B. pensylvanicus’ range (Agricorp 2016a, b).
Declines in populations of this species have been noted in other studies as well. For example, Giles and Ascher (2006) surveyed more than 1200 Bombus in the Black Rock Forest of New York and found no B. pensylvanicus, while Grixti et al. (2009) found that its range had decreased in Illinois and only represented 4.4% of all recent observations compared to 28.1% historically. Cameron et al. (2011b) did not find it across its’ historical northern and eastern range in the United States, estimating a 23% reduction. Bartomeus et al. (2013) also found it to be in significant decline in the northeastern United States, and Richardson et al. (2019) found it to be in decline in Vermont. It was classified as data deficient in a recent 150 year evaluation of Bombus in New Hampshire as there were not enough recent records to be assessed (Jacobson et al. 2018). Colla and Packer (2008) intensively re-surveyed two areas of Ontario in 2004–2006 previously surveyed in 1971–1973 by Macfarlane (1974), and found no B. pensylvanicus out of 1195 Bombus specimens, despite the species previous abundance of 2.5% (range 0–19.9% per site, according to Macfarlane (1974)). Indeed when they expanded their analyses to all of Canada and the United States, Colla et al. (2012) found B. pensylvanicus was still present in only 34% of historically occupied and re-sampled 50 km × 50 km grid cells.
There are concerns with using relative abundance as a calculation to evaluate changes in populations, as it assumes the denominator (all Bombus spp.) does not change over time; i.e. only the species (B. pensylvanicus) being investigated should be changing (Bartomeus et al. 2013; Jacobson et al. 2018). However, we know with Bombus spp. in North America that this is not true, as some species like B. impatiens have actually increased over time, which would make everything else “rarer” compared to it, while other species, like B. affinis, have also decreased, further complicating the comparison (Colla and Packer 2008; Cameron et al. 2011b; Colla et al. 2012; Jacobson et al. 2018). However, other approaches (e.g. using species richness or presence/absence data, comparing to stable species, modeling with all species weighted equally versus by percent or by number of individuals, standardizing survey effort, etc.) also have concerns, and relative abundance is still widely used by many authors (Cameron et al. 2011b; Colla et al. 2012; Bartomeus et al. 2013; Jacobson et al. 2018).
Survey effort may also impact analyses. Area of Occurrence estimates, particularly at the IUCN recommended 2 km cell width, can be underestimated if cells exist where sampling has not occurred but the species exists (Royal Botanic Gardens Kew 2017); as B. pensylvanicus’ range may cover grid squares where no surveys have (re-)occurred, we also used additional methods of determining status.
Relative abundance is a method that can help account for unequal sample sizes, but low search effort or biased surveys may cause differences in results. In this study, the decades beginning in 1927 and 1937 were unusual ones for relative abundance, as the value for the 1927 decade was much lower than the previous one beginning in 1917, before an extreme jump in relative abundance occurred for the next decade starting in 1937. However, the number of unique survey events and average number of bees collected per survey event do not show similar trends (Fig. ESM.4, ESM.5). The number of survey events decreased over the first part of the century, from the 1907–1916 period to the 1937–1946 period (potentially relating to impacts from the two World Wars and the Great Depression), before rising each decade until 1987–1996 where there was a smaller drop, followed by a reversal in the 1997–2006 period and then drastic jump in the 2007–2016 period (Fig. ESM.4). The average number of bees per survey event varied little on a per decade basis in the first ¾ of the century before dipping in the 1977–1986 period and then increasing linearly through the 2007–2016 period (Fig. ESM.5). While the Bumble Bee Watch citizen science program greatly increased the number of total unique events in 2007–2016 as compared to prior decades, and lowered the average number of bees collected per survey event compared to that from the other data sources in the 2007–2016 period (Fig. ESM.6, ESM.7), this variation in effort is unlikely to have affected our analyses.
Regardless of the method used and variation in survey effort, the overall trend over time and comparison of the historic as compared to the recent data (particularly EOO and relative abundance) show that there has been declines in B. pensylvanicus that are cause for concern.
Additionally, this study focused on B. pensylvanicus in Canada, which is an arbitrarily delimited political area. Calculations using data from this region has value particularly for national conservation efforts and assessments. It is possible that the addition of observations from adjacent areas in the United States may change these results. As indicated above, this species has shown to be in decline over its entire geographic range and in studies evaluating its current status. However, further research in the adjacent areas, such as those that do not currently have a Nature Serve ranking (NatureServe 2015), should be conducted to investigate this angle.
As is clear from above, further research into B. pensylvanicus’ biology and threats is required as there is much not yet known. While broad conservation actions for the recovery of Bumble bees in general have been suggested (see e.g. Cameron et al. (2011b) for the final report of the 2010 North American Bumble bee Species Conservation Planning Workshop, and the Ontario Bombus recovery strategies (Environment and Climate Change Canada 2016; Colla 2017), specific needs for this species still need to be identified (Hatfield et al. 2015c).
Bombus affinis co-occurred with B. pensylvanicus in southern Ontario, and was listed as Endangered federally and provincially in 2010 (COSEWIC 2010; COSSARO 2010; Queen’s Printer for Ontario 2016). However, no individuals have been observed throughout its Canadian range since 2009 (Colla and Taylor-Pindar 2011; Government of Ontario 2017) indicating the listing and subsequent protection may have occurred at a date too late to conserve this species from extirpation in Canada. It is imperative that the status of B. pensylvanicus in Canada is determined in order to begin conservation management strategies to avoid the regional extirpation of this species.
Extreme declines in relative abundance and moderate declines in Extent of Occurrence were seen for B. pensylvanicus in its Canadian range, meriting it a suggested IUCN rank of Critically Endangered. Citizen science data provided valuable coverage and can be used in assessments of other species, particularly in conjunction with historic museum specimens and researcher-led field work. Promotion of citizen science programs should therefore occur in order to increase the number of submissions, our knowledge of species, and an interest by the public through government agencies in conserving them. Data from this study can be used to support provincial and federal assessments, encourage habitat restoration and management efforts, effect policy related to commercial pollinators, and guide further research.
Change history
22 May 2019
We thank all museum collections and contributors of bumble bee species occurrence data to the Bumble Bees of North America database managed by LLR (see <ExternalRef><RefSource>http://www.leifrichardson.org/bbna.html</RefSource><RefTarget Address="http://www.leifrichardson.org/bbna.html" TargetType="URL"/></ExternalRef>). We thank everyone who submitted and/or verified observations for the citizen science program Bumble Bee Watch, especially Rich Hatfield of the Xerces Society, and to the partners and funders of that program. We thank Environment and Climate Change Canada, the Ontario Ministry of Natural Resources and Forestry, The W. Garfield Weston Foundation, the Rogers Foundation, the Schad Foundation, and Wildlife Preservation Canada for funding and supporting the field research by VJM and SRC. We acknowledge the support of the Natural Sciences and Engineering Research Council of Canada (NSERC), reference numbers RGPIN-2017-05642 and CGSD-503997-2017. VJM also thanks the <Emphasis Type="Italic">rare</Emphasis> Charitable Research Preserve for a scholarship.
References
Agricorp (2016a) 2016 Corn Harvest at a glance (yield map). http://www.agricorp.com/en-ca/News/2017/Pages/PI-MapsShowAverageCornSoybeanYields.aspx. Accessed 9 July 2018
Agricorp (2016b) 2016 Soybean Harvest at a glance (yield map). http://www.agricorp.com/en-ca/News/2017/Pages/PI-MapsShowAverageCornSoybeanYields.aspx. Accessed 9 July 2018
Agriculture and Agri-Food Canada (2017) Bee Health Roundtable. http://www.agr.gc.ca/eng/industry-markets-and-trade/value-chain-roundtables/bee-health/?id=1409832956249. Accessed 2 Mar 2017
Arbetman MP, Gleiser G, Morales CL et al (2017) Global decline of bumblebees is phylogenetically structured and inversely related to species range size and pathogen incidence. Proc R Soc B Biol Sci 284:20170204. https://doi.org/10.1098/rspb.2017.0204
Bachman S, Moat J, Hill AW et al (2011) Supporting red list threat assessments with GeoCAT: geospatial conservation assessment tool. Zookeys 150:117–126. https://doi.org/10.3897/zookeys.150.2109
Bartomeus I, Ascher JS, Wagner D et al (2011) Climate-associated phenological advances in bee pollinators and bee-pollinated plants. Proc Natl Acad Sci 108:20645–20649. https://doi.org/10.1073/pnas.1115559108
Bartomeus I, Ascher JS, Gibbs J et al (2013) Historical changes in northeastern US bee pollinators related to shared ecological traits. Proc Natl Acad Sci USA 110:4656–4660. https://doi.org/10.1073/pnas.1218503110
Beckham JL, Atkinson S (2017) An updated understanding of Texas bumble bee (Hymenoptera: Apidae) species presence and potential distributions in Texas, USA. PeerJ 5:e3612. https://doi.org/10.7717/peerj.3612
Burkle LA, Marlin JC, Knight TM (2013) Plant-pollinator interactions over 120 years: loss of species, co-occurrence, and function. Science 80(339):1611–1615. https://doi.org/10.1126/science.1232728
Cameron SA, Williams PH, Day L (2001) E-mail communications regarding bee stings. In: Bombus-L List. https://listserv.umd.edu/cgi-bin/wa?A2=ind0104&L=BOMBUS-L&P=R214&I=-3. Accessed 9 Jul 2018
Cameron S, Jepsen S, Spevak E, et al (2011a) North American Bumble Bee Species Conservation Planning Workshop Final Report
Cameron SA, Lozier JD, Strange JP et al (2011b) Patterns of widespread decline in North American bumble bees. Proc Natl Acad Sci USA 108:662–667. https://doi.org/10.1073/pnas.1014743108
Cameron SA, Lim HC, Lozier JD et al (2016) Test of the invasive pathogen hypothesis of bumble bee decline in North America. Proc Natl Acad Sci 113:4386–4391. https://doi.org/10.1073/pnas.1525266113
Cardoso P, Erwin TL, Borges PAV, New TR (2011) The seven impediments in invertebrate conservation and how to overcome them. Biol Conserv 144:2647–2655. https://doi.org/10.1016/j.biocon.2011.07.024
Colla SR (2016) Status, threats and conservation recommendations for wild Bumble Bees (Bombus spp.) in Ontario, Canada: a review for policymakers and practitioners. Nat Areas J 36:412–426. https://doi.org/10.3375/043.036.0408
Colla SR (2017) Recovery strategy for the Gypsy Cuckoo Bumble Bee (Bombus bohemicus) in Ontario. Ontario Recovery Strategy Series, Peterborough, ON
Colla SR, Dumesh S (2010) The Bumble Bees of Southern Ontario: notes on natural history and distribution. J Entomol Soc Ontario 141:39–68. https://doi.org/10.1007/s00442-011-2179-6
Colla SR, MacIvor JS (2016) Questioning public perception, conservation policy, and recovery actions for honeybees in North America. Conserv Biol. https://doi.org/10.1111/cobi.12839.this
Colla SR, Packer L (2008) Evidence for decline in eastern North American bumblebees (Hymenoptera: Apidae), with special focus on Bombus affinis Cresson. Biodivers Conserv 17:1379–1391. https://doi.org/10.1007/s10531-008-9340-5
Colla S, Taylor-Pindar A (2011) Recovery strategy for the rusty-patched Bumble Bee (Bombus affinis) in Ontario. Ontario, Peterborough
Colla SR, Otterstatter MC, Gegear RJ, Thomson JD (2006) Plight of the bumble bee: pathogen spillover from commercial to wild populations. Biol Conserv 129:461–467. https://doi.org/10.1016/j.biocon.2005.11.013
Colla SR, Gadallah F, Richardson L et al (2012) Assessing declines of North American bumble bees (Bombus spp.) using museum specimens. Biodivers Conserv 21:3585–3595. https://doi.org/10.1007/s10531-012-0383-2
Conrad CC, Hilchey KG (2011) A review of citizen science and community-based environmental monitoring: issues and opportunities. Environ Monit Assess 176:273–291. https://doi.org/10.1007/s10661-010-1582-5
COSEWIC (2010) COSEWIC assessment and status report on the rusty-patched bumble bee Bombus affinis in Canda. Committee on the Status of Endangered Wildlife in Canada
COSEWIC (2014a) COSEWIC Assessment and Status Report Gypsy Cuckoo Bumble Bee Bombus bohemicus in Canada. Ottawa, Ontario
COSEWIC (2014b) COSEWIC assessment and status report on the Western Bumble Bee Bombus occidentalis, occidentalis subspecies (Bombus occidentalis occidentalis) and the mckayi subspecies (Bombus occidentalis mckayi) in Canada. Committee on the Status of Endangered Wildlife in Canada
COSEWIC (2015) COSEWIC assessment and status report yellow-banded Bumble Bee (Bombus terricola) in Canada
COSSARO (2010) COSSARO Candidate Species at Risk Evaluation Form for Rusty-patched Bumble Bee (Bombus affinis)
Defenders of Wildlife (2015) A Petition to list the Yellow Banded Bumble Bee (Bombus terricola) as an Endangered, or Alternatively as a Threatened, Species Pursuant to the Endangered Species Act and for the Designation of Critical Habitat for this Species. 1–34
Donovan BJ, Wier SS (1984) Development of hives for field population increase, and studies on the life cycles of the four species of introduced bumble bees in New Zealand. New Zeal J Agric Res 21:733–756. https://doi.org/10.1080/00288233.1978.10427476
Environment and Climate Change Canada (2016) Recovery Strategy for the Rusty-patched Bumble Bee (Bombus affinis) in Canada [Proposed]. Ottawa, Ontario
ESRI (2016) ArcGIS Desktop 10.5
ESTR Secretariat (2016) Mixedwood Plains Ecozone + evidence for key findings summary. Canadian biodiversity: ecosystem status and trends 2010, evidence for key findings summary report no. 7. Canadian Councils of Resource Ministers, Ottawa, Ontario
Follett R, Strezov V (2015) An analysis of citizen science based research: usage and publication patterns. PLoS ONE 10:1–14. https://doi.org/10.1371/journal.pone.0143687
Gaston KJ, Fuller RA (2009) The sizes of species’ geographic ranges. J Appl Ecol 46:1–9. https://doi.org/10.1111/j.1365-2664.2008.01596.x
Gibson SD, Liczner AR, Colla SR (2019) Conservation conundrum: at-risk Bumble Bees (Bombus spp.) show preference for invasive tufted vetch (Vicia cracca) while foraging in protected areas. J Insect Sci. https://doi.org/10.1093/jisesa/iez017
Giles V, Ascher JS (2006) A survey of the bees of the Black Rock Forest Preserve, New York (Hymenoptera: Apoidea). J Hymenopt Res 15:208–231
Gill RJ, Ramos-Rodriguez O, Raine NE (2012) Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 491:105–108. https://doi.org/10.1038/nature11585
Gillespie S (2010) Factors affecting parasite prevalence among wild bumblebees. Ecol Entomol 35:737–747. https://doi.org/10.1111/j.1365-2311.2010.01234.x
Godfray HCJ, Blacquière T, Field LM et al (2014) A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc R Soc B Biol Sci 281:20140558. https://doi.org/10.1098/rspb.2014.0558
Godfray HCJ, Blacquière T, Field LM et al (2015) A restatement of recent advances in the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc R Soc B Biol Sci 282:20151821. https://doi.org/10.1098/rspb.2015.1821
Google (2018) Google Maps. www.google.ca/maps
Goulson D (2015) Neonicotinoids impact bumblebee colony fitness in the field; a reanalysis of the UK’s Food & Environment Research Agency 2012 experiment. PeerJ 3:e854. https://doi.org/10.7717/peerj.854
Goulson D, Nicholls E, Botías C, Rotheray EL (2015) Bee declines driven by combined stress from parasites, pesticides and lack of flowers. Science 80(347):1255957. https://doi.org/10.1126/science.1255957
Government of Ontario (2014) Pollinator health: a proposal for enhancing pollinator health and reducing the use of neonicotinoid pesticides in Ontario. Government of Ontario, Ontario
Government of Ontario (2016) Ontario’s Pollinator Health Action Plan
Government of Ontario (2017) Five-Year Review of Progress Towards the Protection and Recovery of Ontario’s Species at Risk
Graystock P, Blane EJ, McFrederick QS et al (2016) Do managed bees drive parasite spread and emergence in wild bees? Int J Parasitol Parasites Wildl 5:64–75. https://doi.org/10.1016/j.ijppaw.2015.10.001
Grixti JJC, Wong LLT, Cameron SAS, Favret C (2009) Decline of bumble bees (Bombus) in the North American Midwest. Biol Conserv 142:75–84. https://doi.org/10.1016/j.biocon.2008.09.027
Hatfield RG, LeBuhn G (2007) Patch and landscape factors shape community assemblage of bumble bees, Bombus spp. (Hymenoptera: Apidae), in montane meadows. Biol Conserv 139:150–158. https://doi.org/10.1016/j.biocon.2007.06.019
Hatfield R, Colla SR, Jepsen S, et al (2015a) IUCN Assessments for North American Bombus spp. (February 2015 update). 1–56
Hatfield R, Jepsen S, Thorp R, et al (2015d) The IUCN Red List of Threatened Species—Bombus terricola. IUCN Red List Threat Species e.T4493750:18
Hatfield R, Jepsen S, Thorp R, et al (2015b) The IUCN Red List of Threatened Species—Bombus fervidus. IUCN Red List Threat Species e.T21215132A21215225
Hatfield R, Jepsen S, Thorp R, et al (2015c) The IUCN Red List of Threatened Species—Bombus pensylvanicus. IUCN Red List Threat Species e.T2121517:
Health Canada (2017) Pollinator Protection. http://www.hc-sc.gc.ca/cps-spc/pest/agri-commerce/pollinators-pollinisateurs/index-eng.php. Accessed 1 Mar 2017
Hoffmann D, Pettis JS, Neumann P (2008) Potential host shift of the small hive beetle (Aethina tumida) to bumblebee colonies (Bombus impatiens). Insectes Soc 55:153–162. https://doi.org/10.1007/s00040-008-0982-9
Iles DT, Williams NM, Crone EE (2018) Source-sink dynamics of bumble bees in rapidly changing landscapes. J Appl Ecol 9:12. https://doi.org/10.1111/1365-2664.13175
Inouye DW (2008) Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology 89:353–362
IUCN (2012a) IUCN Red List Categories and Criteria - Version 3.1. Gland, Switzerland and Cambridge, United Kingdom
IUCN (2012b) Guidelines for Application of IUCN Red List Criteria at Regional and National Levels: Version 4.0
IUCN Standards and Petitions Subcommittee (2016) Guidelines for Using the IUCN Red List Categories and Criteria. Version 12
Jacobson MM, Tucker EM, Mathiasson ME, Rehan SM (2018) Decline of bumble bees in Northeastern North America, with special focus on Bombus terricola. Biol Conserv 217:437–445. https://doi.org/10.1016/j.biocon.2017.11.026
Johnson SD, Steiner KE (2000) Generalization versus specialization in plant pollination systems. Trends Ecol Evol 15:140–143. https://doi.org/10.1016/s0169-5347(99)01811-x
Kerr JT, Pindar A, Galpern P et al (2015) Climate change impacts on bumblebees converage across continents. Science 80(349):177–180. https://doi.org/10.1126/science.aaa7031
Kevan PG (1975) Forest application of the insecticide fenitrothion and its effect on wild bee pollinators (Hymenoptera: Apoidea) of lowbush blueberries (Vaccinium spp.) in Southern New Brunswick, Canada. Biol Conserv 7:301–309. https://doi.org/10.1016/0006-3207(75)90045-2
Kevan PG, Otis GW, Coffin RH et al (1984) Hazards of carbaryl formulations to caged honeybees (Apis mellifera) foraging on flowering canola (Brassica nupus) in Ontario. Proc Entomol Soc Ontario 115:49–54
Kevan PG, Greco CF, Belaoussoff S (1997) Log-normality of biodiversity and abundance in diagnosis and measuring of ecosystemic health: pesticide stress on pollinators on blueberry heaths. J Appl Ecol 34:1122. https://doi.org/10.2307/2405226
Kjohl M, Anders N, Stenseth NC (2011) Potential effects of climate change on crop pollination. Food and Agriculture Organization of the United Nations (FAO), Rome
Kraus FB, Wolf S, Moritz RFA (2009) Male flight distance and population substructure in the bumblebee Bombus terrestris. J Anim Ecol 78:247–252. https://doi.org/10.1111/j.1365-2656.2008.01479.x
Kremen C, Ullman KS, Thorp RW (2011) Evaluating the quality of citizen-scientist data on pollinator communities. Conserv Biol 25:607–617. https://doi.org/10.1111/j.1523-1739.2011.01657.x
Laverty M, Harder D (1988) The bumble bees of eastern Canada. Can Entomol 120:965–987
León-Cortés JL, Cowley MJR, Thomas CD (1999) Detecting decline in a formerly widespread species: how common is the common blue butterfly Polyommatus icarus? Ecography (Cop) 22:643–650. https://doi.org/10.1111/j.1600-0587.1999.tb00513.x
Lozier JD (2014) Revisiting comparisons of genetic diversity in stable and declining species: assessing genome-wide polymorphism in North American bumble bees using RAD sequencing. Mol Ecol 23:788–801. https://doi.org/10.1111/mec.12636
Lozier JD, Cameron SA (2009) Comparative genetic analyses of historical and contemporary collections highlight contrasting demographic histories for the bumble bees Bombus pensylvanicus and B. impatiens in Illinois. Mol Ecol 18:1875–1886. https://doi.org/10.1111/j.1365-294x.2009.04160.x
Lozier JD, Strange JP, Stewart IJ, Cameron SA (2011) Patterns of range-wide genetic variation in six North American bumble bee (Apidae: Bombus) species. Mol Ecol 20:4870–4888. https://doi.org/10.1111/j.1365-294x.2011.05314.x
Lundin O, Rundlöf M, Smith HG et al (2015) Neonicotinoid insecticides and their impacts on bees: a systematic review of research approaches and identification of knowledge gaps. PLoS ONE 10:1–20. https://doi.org/10.1371/journal.pone.0136928
Lye GC, Osborne JL, Park KJ, Goulson D (2012) Using citizen science to monitor Bombus populations in the UK: nesting ecology and relative abundance in the urban environment. J Insect Conserv 16(5):697–707
Mace GM, Collar NJ, Gaston KJ et al (2008) Quantification of extinction risk: IUCN’s system for classifying threatened species. Conserv Biol 22:1424–1442. https://doi.org/10.1111/j.1523-1739.2008.01044.x
Macfarlane RP (1974) Ecology of Bombinae (Hymenoptera: Apidae) of Southern Ontario, with emphasis on their natural enemies and relationships with flowers. Dissertation, University of Guelph
Macfarlane RP, Patten KD, Royce LA et al (1994) Management potential of sixteen North American bumble bee species. Melanderia 50:1–12
Mcfarland KP, Richardson LL, Zahendra S (2016) Vermont Bumble Bee Survey: Faunal Changes in Vermont, 1900-2014 - Final Report to the Vermont Fish and Wildlife Department. Vermont Cent Ecostudies
Memmott J, Craze PG, Waser NM, Price MV (2007) Global warming and the disruption of plant-pollinator interactions. Ecol Lett 10:710–717. https://doi.org/10.1111/j.1461-0248.2007.01061.x
Miller-Struttmann NE, Geib JC, Franklin JD et al (2015) Functional mismatch in a bumble bee pollination mutualism under climate change. Science 80(249):1541–1544. https://doi.org/10.1126/science.aab0868
Nardone E (2013) The bees of Algonquin Park: a study of their distribution, their community guild structure, and the use of various sampling techniques in logged and unlogged hardwood stands. Dissertation, University of Guelph
NatureServe (2015) NatureServe Explorer: An online encyclopedia of life [web application]. Version 7.1. In: NatureServe, Arlington, Virginia. http://explorer.natureserve.org. Accessed 3 Mar 2017
Ontario Biodiversity Council (2010) State of Ontario’s biodiversity 2010 - highlights report. A report of the Ontario Biodiversity Council, Ontario Biodiversity Council, Peterborough, Ontario
Ontario Biodiversity Council (2011) Ontario’s Biodiversity Strategy, 2011: Renewing Our Commitment to Protecting What Sustains Us. Ontario Biodiversity Council, Peterborough, Ontario
Otterstatter MC, Thomson JD (2008) Does pathogen spillover from commercially reared bumble bees threaten wild pollinators. PLoS ONE. https://doi.org/10.1371/journal.pone.0002771
Parliament of Canada (2015) The Importance of Bee Health to Sustainable Food Production in Canada. https://sencanada.ca/Content/SEN/Committee/412/agfo/rep/rep09may15-e.htm. Accessed 2 Mar 2017
Potts SG, Biesmeijer JC, Kremen C et al (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25:345–353. https://doi.org/10.1016/j.tree.2010.01.007
Pyke GH, Thomson JD, Inouye DW, Miller TJ (2016) Effects of climate change on phenologies and distributions of bumble bees and the plants they visit. Ecosphere 7:1–19. https://doi.org/10.1002/ecs2.1267
Queen’s Printer for Ontario (2016) O. Reg. 230/08: Species At Risk in Ontario List
Richardson L, McFarland K, Zahendra S, Hardy S (2019) Bumble bee (Bombus) distribution and diversity in Vermont. A century of change. J Insect Conserv 23:45. https://doi.org/10.1007/s10841-018-0113-5
Rodrigues ASL, Pilgrim JD, Lamoreux JF et al (2006) The value of the IUCN Red List for conservation. Trends Ecol Evol 21:71–76. https://doi.org/10.1016/j.tree.2005.10.010
Royal Botanic Gardens Kew (2017) GeoCAT FAQ 4.9 Does the EOO value exclude “discontinuities or disjunctions” e.g. areas of obviously unsuitable habitat? https://www.kew.org/geocat-faqs#4. Accessed 1 Jun 2017
Schweitzer D, Capuano N, Young B, Colla S (2012) Conservation and management of North American Bumble Bees. NatureServe and the U.S. Forest Service, Washington, DC, pp 1–18
Silvertown J (2009) A new dawn for citizen science. Trends Ecol Evol 24:467–471. https://doi.org/10.1016/j.tree.2009.03.017
Statistics Canada (2016) Table 051-0001 - Estimates of population, by age group and sex for July 1, Canada, provinces and territories, annual (persons unless otherwise noted), CANSIM (database). http://www5.statcan.gc.ca/cansim/a26. Accessed 3 Mar 2017
Szabo ND, Colla SR, Wagner DL et al (2012) Do pathogen spillover, pesticide use, or habitat loss explain recent North American bumblebee declines? Conserv Lett 5:232–239. https://doi.org/10.1111/j.1755-263x.2012.00234.x
Szymanski J, Smith T, Horton A, et al (2016) Rusty Patched Bumble Bee (Bombus affinis) Species Status Assessment, final Report, Version 1. US Fish Wildl Serv
Tang J, Wice J, Thomas VG, Kevan PG (2007) Assessment of Canadian federal and provincial legislation to conserve native and managed pollinators. Int J Biodivers Sci Manag 3:46–55
The Xerces Society for Invertebrate Conservation, Wildlife Preservation Canada, York University, et al (2017) Bumble Bee Watch, a collaborative website to track and conserve North America’s bumble bees. http://www.bumblebeewatch.org
The Xerces Society for Invertebrate Conservation, Wildlife Preservation Canada, York University, et al (2018) Data accessed from Bumble Bee Watch, a collaborative website to track and conserve North America’s bumble bees. Accessed January 31, 2018
van der Wal R, Anderson H, Robinson A et al (2015) Mapping species distributions: a comparison of skilled naturalist and lay citizen science recording. Ambio 44:S584–S600. https://doi.org/10.1007/s13280-015-0709-x
Whitehorn PPR, O’Connor S, Wackers FLF, Goulson D (2012) Neonicotinoid pesticide reduces Bumble Bee colony growth and queen production. Science 80(336):351–352. https://doi.org/10.1126/science.1215025
Williams PH (1988) Habitat use by bumblebees (Bombus spp.). Ecol Entomol 13:223–238
Williams PH, Araújo MB, Rasmont P (2007) Can vulnerability among British bumblebee (Bombus) species be explained by niche position and breadth? Biol Conserv 138:493–505. https://doi.org/10.1016/j.biocon.2007.06.001
Williams P, Thorp R, Richardson L, Colla S (2014) Bumble Bees of North America. Princeton University Press, Princeton, New Jersey
Wood TJ, Goulson D (2017) The Environmental Risks of neonicotinoid pesticides: a review of the evidence post-2013. Environ Sci Pollut Res. https://doi.org/10.1101/098897
Wyatt BK (1970) Ecology of Bumble Bees in Southern Ontario with Reference to Nest Development and Pollen Sources. Dissertation, University of Guelph
Yalden PE (1982) The effect of latitude on colony size in Bombus monticola Smith and B. lapponicus (Fabricius) (Hym., Apidae). Fauna Nor Ser B 29:72–73
Zayed A, Packer L (2005) Complementary sex determination substantially increases extinction proneness of haplodiploid populations. Proc Natl Acad Sci USA 102:10742–10746. https://doi.org/10.1073/pnas.0502271102
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
MacPhail, V.J., Richardson, L.L. & Colla, S.R. Incorporating citizen science, museum specimens, and field work into the assessment of extinction risk of the American Bumble bee (Bombus pensylvanicus De Geer 1773) in Canada. J Insect Conserv 23, 597–611 (2019). https://doi.org/10.1007/s10841-019-00152-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-019-00152-y