Abstract
Background
Ablation of autonomic ectopy-triggering ganglionated plexuses (ET-GP) has been used to treat paroxysmal atrial fibrillation (AF). It is not known if ET-GP localisation is reproducible between different stimulators or whether ET-GP can be mapped and ablated in persistent AF. We tested the reproducibility of the left atrial ET-GP location using different high-frequency high-output stimulators in AF. In addition, we tested the feasibility of identifying ET-GP locations in persistent atrial fibrillation.
Methods
Nine patients undergoing clinically-indicated paroxysmal AF ablation received pacing-synchronised high-frequency stimulation (HFS), delivered in SR during the left atrial refractory period, to compare ET-GP localisation between a custom-built current-controlled stimulator (Tau20) and a voltage-controlled stimulator (Grass S88, SIU5). Two patients with persistent AF underwent cardioversion, left atrial ET-GP mapping with the Tau20 and ablation (Precision™, Tacticath™ [n = 1] or Carto™, SmartTouch™ [n = 1]). Pulmonary vein isolation (PVI) was not performed. Efficacy of ablation at ET-GP sites alone without PVI was assessed at 1 year.
Results
The mean output to identify ET-GP was 34 mA (n = 5). Reproducibility of response to synchronised HFS was 100% (Tau20 vs Grass S88; [n = 16] [kappa = 1, SE = 0.00, 95% CI 1 to 1)][Tau20 v Tau20; [n = 13] [kappa = 1, SE = 0, 95% CI 1 to 1]). Two patients with persistent AF had 10 and 7 ET-GP sites identified requiring 6 and 3 min of radiofrequency ablation respectively to abolish ET-GP response. Both patients were free from AF for > 365 days without anti-arrhythmics.
Conclusions
ET-GP sites are identified at the same location by different stimulators. ET-GP ablation alone was able to prevent AF recurrence in persistent AF, and further studies would be warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Atrial fibrillation (AF) is triggered by ectopic beats from the pulmonary veins (PVs) [1]. Several studies have shown that such PV ectopy can be generated by stimulating the left atrial intrinsic cardiac autonomic nervous system [2,3,4,5,6]. These studies imply that the atrial neural network is important in the pathogenesis of AF. Analysis of the histology of this network in post-mortem human hearts demonstrated dense clusters of nerves in the atrial epicardium called ganglionated plexuses (GP) [2]. These sites could be targeted for ablation by an anatomical approach alone. However, this would be more difficult to control between operators and varying atrial anatomy. The use of high-frequency stimulation (HFS) to identify GP sites is reproducible and gives a clear endpoint for ablation. The first functional studies of this network in patients identified GP sites exhibiting atrioventricular dissociation (AVD) in response to several seconds of continuous high-frequency stimulation (HFS) [3,4,5,6]. Subsequently, ectopy-triggering (ET) sites were also located by a different method delivering short bursts of HFS during the atrial refractory period and observing for a triggered ectopic response [7, 8]. Global left atrial GP mapping has been used to create anatomical maps of the locations of GPs and whilst there is anatomical overlap of AVD and ET GPs in a proportion of cases this is not complete [9]. This has encouraged the use of separate HFS mapping approaches. ET-GP can only be located in sinus rhythm, and AF induced by ET-GP stimulation can sometimes become resistant to cardioversion precluding further ET-GP mapping. Consequently, it has been assumed that ET-GP mapping would be futile during persistent AF and the distribution of ET-GP in patients with persistent AF is not known.
The GANGLIA-AF trial showed that when the entire atrial ET-GP could be mapped and ablated in patients with paroxysmal AF, freedom from atrial arrhythmias was similar to a standard pulmonary vein isolation procedure [8, 10, 11]. The trial used the Grass S88 stimulator which is not licenced for routine clinical work but has been used in a wide variety of autonomic research studies. It is not known whether the localisation of ET-GP sites is in any way stimulator specific. We designed and built a custom stimulator (Tau20), meeting IEC 60,601–1 3rd Edition Regulatory Standards, with similar HFS delivery profile to the Grass stimulator. Following bench and animal testing, approval was granted for a comparative study of the efficacy and reproducibility of this current-controlled stimulator (Tau20) against the voltage-controlled Grass stimulator with SIU5 in patients undergoing paroxysmal AF ablation. Feasibility of mapping ET-GP in persistent AF was also tested using the Tau20 stimulator in order to justify a larger study looking at identification and ablation of ET-GP sites in persistent AF.
2 Methods
Patients undergoing clinically indicated AF ablation consented to participate. All cases were performed under general anaesthetic. Patients were excluded if they were unable to provide informed consent, developed a contraindication to catheter ablation (e.g. presence of cardiac thrombus), valvular disease moderate or greater or EF < 30%. Reproducibility of response was analysed using Cohen’s kappa test at the location level to assess within patient agreement. The research was approved by the NHS Research Ethics Committee (REC) London-Fulham Ref:14/LO/2044 and 17/LO/0465, and the South central-Berkshire 20/SC/0081.
2.1 The Grass S88 stimulator
The Grass S88 stimulator has been widely used for autonomic studies. It has two primary output channels (S1 and S2). The S1 outputs stimuli to pace the atrium and S2 delivers short and repeated bursts of (HFS) synchronised to the paced stimuli from S1. Each channel is connected to an isolation unit (SIU5), which converts the Grass output to isolated constant voltage.
2.2 The Tau20
The Tau20 is a current-controlled high-frequency stimulator. Figure 1 illustrates the simplified model of Tau20 when delivering constant amplitude current pulses to the tissue (range 1–100 mA).
A simplified model of the Tau20 setup. The output impedance (ROUT,TAU, > 300 KΩ by specification) of the Tau20 is much higher than the tissue impedance (RTISSUE ~ 0.2 KΩ based on Langendorff observations) resulting in the stimulating current (ITAU,OUT) being delivered practically in totality to the tissue. Internal Tau20 circuitry prevents the output voltage (VT,TAU) developed across the tissue (typically during HFS stimulation, VT,TAU is the differential voltage across MAP1–2) from exceeding 15 V
2.3 Identification of ET-GP
ET-GP can only be identified during sinus rhythm. HFS was delivered to the left atrial endocardium via a standard electrode catheter (Navistar™ and Tacticath™), synchronised to a paced train to deliver HFS within the atrial refractory period (80–160-ms duration at 40 Hz, 10 V, up to 15 trains). This ensures ET-GP (not atrial tissue) are specifically recruited. A positive response was atrial or PV ectopy and/or AF [9, 10, 12, 13]. Test sites were marked on the left atrial geometry created using commercially available electroanatomic mapping systems (CARTO™ & Precision™). Previous studies [8, 9] have indicated that complete GP mapping of the left atrium prior to therapeutic ablation adds roughly 60 min to the total procedure time. We elected not to perform mapping of the whole left atrium in these validation cases.
2.4 Threshold testing for ET-GP response using Tau20
ET-GP mapping was performed using the Tau20 at an HFS output of 50 mA. The threshold for this response was determined by reducing the output by 10 mA increments until the effect was lost. The threshold at which the response was regained was assessed by increasing the output from 10 mA.
2.5 Reproducibility of ET-GP identification by Tau20
A left atrial ET-GP map was created using the Tau20 at an output of 50 mA and then repeating HFS at the same sites with the same parameters to see if the same functional response was identified. Reproducibility of the Grass stimulator against itself has previously been validated [6, 9, 12].
2.6 Reproducibility of ET-GP identification between Tau20 and Grass stimulator
A left atrial ET-GP map was created with one stimulator and the same locations tested with the alternative stimulator (Tau20 and Grass S88 with SIU5) to assess reproducibility. Tau20 output was 50 mA and the Grass S88 output 100 V (equivalent to ~ 10 V at the tissue). Reproducibility of both positive and negative responses was calculated.
2.7 Feasibility testing of ET-GP mapping and ablation in persistent AF
Two patients undergoing ablation for persistent AF were recruited. A summary of the demographics of these patients can be seen in Table 1. The procedure was performed under general anaesthetic with interrupted anticoagulation and trans-oesophageal echo to confirm no left atrial appendage thrombus. Direct current external cardioversion with concomitant amiodarone use was planned to maintain sinus rhythm. The Tau20 was used to map ET-GP with synchronised HFS via the Navistar™ or Tacticath™ catheters. ET-GP sites were ablated immediately and retested to ensure loss of response. Anatomical test locations were marked on the 3D anatomy using CARTO™ or Precision™. No PVI was performed. The patient was followed up with clinic visits and Holters for 1 year to assess recurrence of AF.
3 Results
Nine patients with paroxysmal AF were recruited for this part of the study. Table 1 summarises patient demographics for this study, and Table 2 summarises which research protocol was applicable to each patient.
3.1 Threshold assessment
The mean threshold for ET-GP was 34 mA (n = 5 sites in 5 patients). The threshold for eliciting a positive response was patient and site specific with a range from 20 to 50 mA. Fifty milliamperes was therefore selected as the current output for subsequent reproducibility assessment to ensure optimal GP detection.
3.2 Reproducibility of GP identification between Tau20 and Grass S88 with SIU5
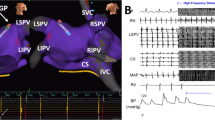
Sixteen synchronised HFS test sites (n = 2 patients) were compared between the Tau 20 and Grass stimulator. Reproducibility was 100% (positive, Fig. 2, or negative, Fig. 3) (kappa = 1, SE of kappa = 0.00, 95% CI 1 to 1).
Reproducibility of ET-GP sites identified with synchronised HFS using the Tau20 compared to Grass stimulator. The left atrial anatomy (grey) as visualised with the CARTO™ electroanatomic mapping system. Electrogram (EGM) signals from the EP recording system (LabSystem Pro, Boston Scientific) are shown adjacent. ET-GP sites green. Ectopy circled in red solid lines. HFS applied endocardially via the Map catheter from the Grass stimulator with SIU5 (output 100 V) and then the Tau20 (output 50 mA). Arterial line trace top, CS EGMs, MAP EGMs, PV EGMs bottom
Reproducibility of HFS negative sites with synchronised HFS using the Tau20 compared to Grass stimulator. The left atrial anatomy (grey) as visualised with the CARTO™ electroanatomic mapping system. Electrogram signals from the EP recording system (LabSystem Pro, Boston Scientific) are shown adjacent. Negative synchronised HFS test sites in dark blue/purple. HFS applied endocardially via the Map catheter from the Grass stimulator with SIU5 (output 100 V) and then the Tau20 (output 50 mA). Coronary sinus EGMs above, Map catheter EGMs below
3.3 Reproducibility of GP identification by Tau20
Thirteen synchronised HFS test sites (n = 4 patients) were tested multiple times using the Tau20 to ensure repeatable identification of ET-GP sites using the Tau20 stimulator. Reproducibility was 100% (kappa = 1, SE of kappa = 0, 95% CI 1 to 1).
3.4 Feasibility of ET-GP mapping and ablation in persistent AF
Two patients with persistent AF were recruited. Patient 1 had AF lasting 33 months following an initial successful external cardioversion. The left atrial diameter was 4.5 cm with preserved left ventricular function. Patient 2 had persistent AF for 4 months before external cardioversion lasting 2 days. A subsequent cardioversion was performed after 9 months of AF on amiodarone and remained in sinus rhythm until the ablation procedure. The left atrial diameter was 4.2 cm with normal left ventricular function.
Patient 1 had 114 sites tested with synchronised HFS from the Tau20 via the Tacticath™ catheter and recorded on the Precision™ system, identifying 10 ET-GP sites (9% of total) (Fig. 4). Each ET-GP was ablated following identification (11 lesions, 9052 J, 6 min 12 s RF duration) and confirmed by negative response to HFS on retesting the site.
The Tau20 can be used to complete a high-density ET-GP map in a patient with persistent AF in Precision™. Main picture: the left atrial anatomy (grey) as visualised with the Precision™ electroanatomic mapping system. HFS applied endocardially via the Map catheter from the Tau20 (output 50 mA). ET-GP (green), HFS-negative sites (blue), incidental phrenic nerve capture (orange). Second picture: HD grid located in the right lower pulmonary vein (RLPV). Coronary sinus EGMs top, Map catheter middle, HD grid EGMs bottom. Ectopy circled in red solid lines
Patient 2 had 124 sites tested with synchronised HFS from the Tau20 via the SmartTouch™ catheter and recorded on the CARTO™ system, identifying 7 ET-GP (6%) (Fig. 5). Again, each ET-GP was ablated following identification (7 lesions, 4853 J, 2 min 58 s RF duration) and confirmed with a negative response in retesting.
The Tau 20 can be used to identify ET-GP in a patient with persistent AF to guide therapeutic ablation. The left atrial anatomy (grey) as visualised with the Carto™ electroanatomic mapping system. HFS applied endocardially via the Map catheter from the Tau20 (output 50 mA). ET-GP (green), HFS-negative sites (purple). Ablation lesions (red). CS EGMs top, MAP EGMs middle, PV EGMs bottom. Ectopy circled in red solid lines. EGMs left are pre-ablation, and EGMs right are the same sites post ablation confirming efficacy of ablation
There were no complications in either case. All antiarrhythmics were stopped after the 3-month blanking period. Both patients underwent regular Holter recordings and ECGs. There was no recurrence of AF following the 12-month review.
4 Discussion
This is the first study to confirm that ET-GP can be reproducibly located at the same site by two different stimulators. We used the Tau20 to perform a first-in-man study to show that ET-GP can be identified in patients with persistent AF and provide proof-of-concept that ET-GP ablation alone without pulmonary vein isolation could prevent AF in persistent AF.
The role of the autonomic nervous system in arrhythmia and AF is well known and illustrated in a number of animal studies [6, 14, 15]. The cardiac autonomic nervous system has been assessed as a therapeutic target for AF ablation with mixed results [13, 16, 17]. GANGLIA-AF demonstrated that ablation of GP sites alone can prevent AF recurrence in about 50% of patients with paroxysmal AF [10, 11]. Importantly, this was the first study to specifically target ET-GP rather than AVD-GP. Functional autonomic work in patients with paroxysmal AF has demonstrated that AVD-GP and ET-GP have different anatomical distributions within the atrium [18]. This highlights the importance of functional testing to identify the location of GP and may account for differential success rates in targeted GP ablation. Mapping of ET-GP requires maintenance of SR to enable detection of ectopy. We have demonstrated that it is possible to maintain SR to use synchronised HFS to create a detailed ET-GP map in patients with persistent AF.
It has been suggested that inadvertent ablation of GP during circumferential PV antral ablation may explain the observation of freedom from AF despite PV reconnection [19]. ET-GP are likely to be preferentially ablated compared to AVD-GP during CPVA due to their anatomical distribution, resulting in treatment of the upstream trigger for AF. However, PVI is known to be less successful in patients with persistent AF as compared to paroxysmal AF [20] so non-PV triggers are of greater mechanistic and therapeutic interest. ET-GP are able to trigger PV and atrial body ectopy from sites distant to the PV and outside the line of CPVA. It is therefore important that ET-GP can be selectively mapped and ablated in persistent AF as demonstrated in this proof-of-concept study using the Tau20. The results of this 2 patient pilot study, showing the successful mapping and ablation of ET-GP in patients with persistent AF, with freedom from AF at 1 year, prove that GP mapping is not only feasible in this cohort of patients, but also worthy of significant further investigation.
Ablation of targets in addition to PVI is common in the treatment of persistent AF. This can lead to significant amounts of RF ablation in atria that often already have a high burden of scar [21]. The ability to map GP, tailored to the ET-GP sub-group, in persistent AF enabled the two patients presented here to have very limited ablation. A larger study is required to see if this limited ablation in persistent AF can achieve similar outcomes to the GANGLIA-AF study which achieved freedom from AF with up to 56% the amount of RF energy used in conventional PVI.
PV isolation has been the cornerstone of AF ablation since the early 2000s but despite significant advances in techniques to enable durable PVI, further improvement in outcomes is needed [22, 23]. That GP ablation (without PVI) can prevent AF recurrence [11] which implies GP sites are part of the AF mechanism even in persistent AF and so should be investigated further as a potential adjunct to improve outcomes. This could be in combination with or post PVI (at a first time or a redo procedure respectively). To minimise additional procedure time, high-density GP mapping could be limited to identification of non-PV triggers outside the PV antrum or combined with single-shot PVI technology.
5 Limitations
We compared only two stimulators and we do not know if this reproducibility extends to other commercially available stimulators. The Grass stimulator has been the most widely used for autonomic studies and the Tau20 was designed to have a similar performance whilst meeting current regulatory standards. However, should similar parameters be used in further studies, then similar results would be expected.
A further limitation is the small sample size of this study. Whilst the size is adequate to ensure that the Tau20 performs consistently and to an equal or better standard than the Grass stimulator, further studies into the efficacy of mapping and ablation of ET-GP in persistent AF are warranted before any definitive conclusions can be drawn. This study does provide compelling evidence that a further study into this field is warranted.
6 Conclusion
ET-GP sites can be located with a high degree of reproducibility by different stimulators. ET-GP sites can be identified in persistent AF and ablation of ET-GP can prevent recurrence of persistent AF.
References
Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339(10):659–66.
Armour JA, Murphy DA, Yuan BX, Macdonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec. 1997;247(2):289–98.
Scanavacca M, Pisani CF, Hachul D, Lara S, Hardy C, Darrieux F, et al. Selective atrial vagal denervation guided by evoked vagal reflex to treat patients with paroxysmal atrial fibrillation. Circulation. 2006;114(9):876–85.
Malcolme-Lawes LC, Lim PB, Wright I, Kojodjojo P, Koa-Wing M, Jamil-Copley S, et al. Characterization of the left atrial neural network and its impact on autonomic modification procedures. Circ Arrhythm Electrophysiol. 2013;6(3):632–40.
Po SS, Nakagawa H, Jackman WM. Localization of left atrial ganglionated plexi in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20(10):1186–9.
Nakagawa H, Scherlag BJ, Patterson E, Ikeda A, Lockwood D, Jackman WM. Pathophysiologic basis of autonomic ganglionated plexus ablation in patients with atrial fibrillation. Heart Rhythm. 2009;6(12 Suppl):S26-34.
Lim PB, Malcolme-Lawes LC, Stuber T, Wright I, Francis DP, Davies DW, et al. Intrinsic cardiac autonomic stimulation induces pulmonary vein ectopy and triggers atrial fibrillation in humans. J Cardiovasc Electrophysiol. 2011;22(6):638–46.
Kim M-Y, Lim PB, Coyle C, Sandler B, Koa-Wing M, Kanagaratnam P. Single ectopy-triggering ganglionated plexus ablation without pulmonary vein isolation prevents atrial fibrillation. JACC: Case Reports. 2020;2(12):2004–9.
Kim MY, Sikkel MB, Hunter RJ, Haywood GA, Tomlinson DR, Tayebjee MH, et al. A novel approach to mapping the atrial ganglionated plexus network by generating a distribution probability atlas. J Cardiovasc Electrophysiol. 2018;29(12):1624–34.
Sandler B, Kim MY, et al. Targeting the ectopy-triggering ganglionated plexuses without pulmonary vein isolation prevents atrial fibrillation. J Cardiovasc Electrophysiol. 2021;32(2):235–44.
Kim MY, et al. Ectopy-triggering ganglionated plexuses ablation to prevent atrial fibrillation: GANGLIA-AF study. Heart Rhythm. 2022;19(4):516–24.
Lemery R, Birnie D, Tang AS, Green M, Gollob M. Feasibility study of endocardial mapping of ganglionated plexuses during catheter ablation of atrial fibrillation. Heart Rhythm. 2006;3(4):387–96.
Driessen AHG, Berger WR, Krul SPJ, van den Berg NWE, Neefs J, Piersma FR, et al. Ganglion plexus ablation in advanced atrial fibrillation: the AFACT study. J Am Coll Cardiol. 2016;68(11):1155–65.
Lu Z, Scherlag BJ, Lin J, Niu G, Fung KM, Zhao L, et al. Atrial fibrillation begets atrial fibrillation: autonomic mechanism for atrial electrical remodeling induced by short-term rapid atrial pacing. Circ Arrhythm Electrophysiol. 2008;1(3):184–92.
Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS Jr, Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res. 1991;68(5):1471–81.
Pokushalov E, Romanov A, Katritsis DG, Artyomenko S, Shirokova N, Karaskov A, et al. Ganglionated plexus ablation vs linear ablation in patients undergoing pulmonary vein isolation for persistent/long-standing persistent atrial fibrillation: a randomized comparison. Heart Rhythm. 2013;10(9):1280–6.
Katritsis DG, Pokushalov E, Romanov A, Giazitzoglou E, Siontis GC, Po SS, et al. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Cardiol. 2013;62(24):2318–25.
Kim MY, Sandler B, Sikkel MB, Cantwell CD, Leong KM, Luther V, et al. The ectopy-triggering ganglionated plexuses in atrial fibrillation. Auton Neurosci. 2020;228:102699.
Nery PB, Belliveau D, Nair GM, Bernick J, Redpath CJ, Szczotka A, et al. Relationship Between pulmonary vein reconnection and atrial fibrillation recurrence: a systematic review and meta-analysis. JACC Clin Electrophysiol. 2016;2(4):474–83.
Weinmann K, Aktolga D, Pott A, Bothner C, Rattka M, Stephan T, et al. Impact of re-definition of paroxysmal and persistent atrial fibrillation in the 2012 and 2016 European Society of Cardiology atrial fibrillation guidelines on outcomes after pulmonary vein isolation. J Interv Card Electrophysiol. 2021;60(1):115–23.
Adeola O VD, Subash Shantha G, et al. Association between left atrial scar burden and persistent versus paroxysmal atrial fibrillation: a retrospective cohort study. J Am Coll Cardiol. 2018; 71
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498.
Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Europace. 2018;20(1):157–208.
Funding
The research leading to these results received funding from The British Heart Foundation under CRTF grant FS/20/14/34917. Partial financial support was received from The British Cardiac Trust, St Mary’s Coronary Flow Trust, and Imperial Confidence in Concept Scheme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The research was approved by the NHS Research Ethics Committee (REC) London-Fulham Ref: 14/LO/2044 and 17/LO/0465, and the South Central-Berkshire 20/SC/0081.
Consent to participate
Informed consent to participate in this study, and to have their study data included in the publication, was freely given in writing by all patients enrolled in the study.
Conflict of interest
Intellectual Property relating to this field was filed by Imperial College on behalf of SK, ED, NL, and PK. No other COI is disclosed.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We can confirm the manuscript is not under consideration elsewhere, and none of the paper’s contents has been previously published.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Coyle, C., Koutsoftidis, S., Kim, MY. et al. Feasibility of mapping and ablating ectopy-triggering ganglionated plexus reproducibly in persistent atrial fibrillation. J Interv Card Electrophysiol (2023). https://doi.org/10.1007/s10840-023-01517-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10840-023-01517-9