Abstract
Background
We describe our initial experience using a multipolar pulsed-field ablation catheter for the treatment of left atrial (LA) reentry tachycardia.
Methods
We included all patients with LA reentry tachycardia treated with PFA at our institution between September 2021 and March 2022. The tachycardia mechanism was identified using 3D electro-anatomical mapping (3D-EAM). Subsequently, a roof line, anterior line, or mitral isthmus line was ablated as appropriate. Roof line ablation was always combined with LA posterior wall (LAPW) ablation. Positioning of the PFA catheter was guided by a 3D-EAM system and by fluoroscopy. Bidirectional block across lines was verified using standard criteria. Additional radiofrequency ablation (RFA) was used to achieve bidirectional block as necessary.
Results
Among 22 patients (median age 70 (59–75) years; 9 females), we identified 27 LA reentry tachycardia: seven roof dependent macro-reentries, one posterior-wall micro-reentry, twelve peri-mitral macro-reentries, and seven anterior-wall micro-reentries. We ablated a total of 20 roof lines, 13 anterior lines, and 6 mitral isthmus lines. Additional RFA was necessary for two anterior lines (15%) and three mitral isthmus lines (50%). Bidirectional block was achieved across all roof lines, 92% of anterior lines, and 83% of mitral isthmus lines. We observed no acute procedural complications.
Conclusion
Ablation of a roof line and of the LAPW is feasible, effective, and safe using this multipolar PFA catheter. However, the catheter is less suited for ablation of the mitral isthmus and the anterior line. A focal pulsed-field ablation catheter may be more effective for ablation of these lines.
Graphical Abstract
This study shows the feasibility to ablate linear lesions with a multipolar pulsed-field ablation catheter. 27 left atrial reentry tachycardia were treated in 22 patients.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Reentrant left atrial (LA) tachycardia may develop following LA ablation of atrial fibrillation or occur spontaneously in diseased LA. The main mechanisms of reentrant LA tachycardia are roof-dependent- or mitral isthmus-dependent macro-reentry, or micro-reentry involving scar tissue.
The ablation strategy for LA tachycardia involves ablation of a line across the roof, the mitral isthmus or anterior wall, or focal ablation of scar tissue. However, in 20–30% of LA linear ablations, bidirectional block cannot be achieved acutely. Even if bidirectional block can be achieved, it may recover in up to 40% of cases. Both scenarios can facilitate new arrhythmias [1,2,3]. Most operators use radiofrequency (RF) energy for LA linear ablations. However, RF energy must be used cautiously, particularly on the left atrial posterior wall (LAPW), to avoid serious side effects such as esophageal damage or perforation. Different strategies have been adopted to overcome the limitations of RF energy. The most successful strategy may be ethanol injection into the vein of Marshal for mitral isthmus line ablation or adjunctive epicardial ablation for roof lines [4,5,6,7]. However, these adjunctive approaches are time-consuming and limited to experienced centers.
Pulsed field ablation (PFA) is a novel, non-thermal ablation modality in which cardiac tissue is exposed to repetitive short and intense electrical fields [8]. PFA results in destabilization of cell membranes, formation of pores, and ultimately cell death. Cardiac muscle cells are particularly vulnerable to this effect resulting in selective ablation of the myocardium while sparing other tissues [9]. PFA therefore holds the promise of increased efficacy without sacrificing safety. The first PFA catheter received CE mark in 2021 and is available for clinical use in Europe. The design of this PFA catheter is optimized for pulmonary vein isolation (PVI), but other LA arrhythmias may also be targeted. We report our first experience using a novel pentaspline PFA catheter for LA reentrant tachycardia.
2 Methods
2.1 Study population
Consecutive patients with documented LA reentry tachycardia undergoing ablation by PFA at our institution were prospectively enrolled into an institutional registry. The registry was approved by the local ethics committee (approval number PB_2018-00,226), and the study was carried out in accordance with the principles of the Declaration of Helsinki. The authors had full access to and take full responsibility for the integrity of the data.
2.2 Procedural workflow
Our workflow has been described previously [10]. In brief, all patients underwent pre-procedural trans-esophageal echocardiography and cardiac computed tomography to rule out intracardiac thrombi and to obtain a detailed 3D anatomy of the LA. Deep conscious sedation was induced by midazolam, fentanyl, and propofol in an operator-directed, nurse-administered fashion [11]. No paralytics were used. LA access was obtained either by transseptal puncture or through a patent foramen ovale. Heparin was administered to maintain an activated clotting time above 350 s during the procedure. Subsequently, the standard sheath used for LA access was exchanged for a dedicated 13-F steerable sheath (Faradrive, Boston Scientific, Marlborough, MA). Next, a multipolar 3D-mapping catheter (Pentaray, Biosense Webster, Irvine, CA) was introduced through the 13-F steerable sheath. With this catheter, a 3D electroanatomical map (3D-EAM) of the LA was constructed, and the tachycardia mechanism was determined. Reentry mechanisms suitable for ablation by PFA were defined as roof-dependent or peri-mitral macro-reentry tachycardia or scar-related anterior or posterior micro-reentry tachycardia. The mapping catheter was then exchanged for the multipolar PFA catheter, and the pulmonary veins (PVs) were isolated as necessary [12]. Additional ablations were applied to create appropriate linear lesions to target the reentrant tachycardia. In the case of roof-dependent reentry, we performed a roof line, followed by complete LAPW isolation. For anterior scar-related reentry or peri-mitral macro-reentry with an extensive anterior scar, an anterior line was ablated by placing lesions between a superior pulmonary vein and the high anterior mitral annulus. For peri-mitral macro-reentry without an extensive anterior scar, we ablated a standard mitral isthmus line from the left inferior pulmonary vein (LIPV) to the posterior mitral annulus. PVI was confirmed by testing for bidirectional block.
2.3 Ablation system
The PFA platform, called the FARAPULSE PFA System (Boston Scientific, Marlborough, Massachusetts), includes a generator (Farastar), which produces the therapeutic electrical field, a 12F over-the-wire multipolar ablation catheter (Farawave), available in two sizes (31 mm and 35 mm), and a 13-F steerable sheath (Faradrive). The platform has been described in detail previously and is CE Marked with an indication for PVI in patients with paroxysmal atrial fibrillation [8]. The multipolar ablation catheter has five splines containing four electrodes each. Intracardiac electrograms can be recorded in between splines from the third-to-distal electrode of each spline. The five splines join at their proximal and distal end and can take any intermediary form between a spherical “basket” shape to a fully-deployed “flower” configuration. For ablation, microsecond-scale, high-amplitude electrical pulses are applied between all 20 electrodes in a bipolar, biphasic fashion. This creates a complex electrical field around the catheter and results in irreversible electroporation of any susceptible cells within this field. We chose a peak voltage of 2.0 kV for all ablations and used the 31 mm or 35 mm device according to operator choice.
2.4 Visualization of the PFA catheter on the 3D-EAM system
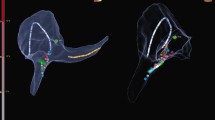
For 3D-EAM, we used both the Carto (Carto 3, Biosense Webster, Irvine, CA) and Rhythmia system (Boston Scientific, Marlborough, MA). Visualization of the PFA catheter in the Carto system was realized by defining a custom 6F circular catheter with six 2-mm electrodes and 17-mm center-to-center inter-electrode spacing. Since the catheter only has five splines, we split the signal of the first electrode and routed it to the additional sixth pin, consequently displaying the curved shape always as a closed loop. Catheter position was verified on 3D-EAM and fluoroscopy before ablation (Figs. 1 and 2). The location pad of the 3D-mapping system was disabled during ablation to speed up visualization recovery thereafter. Visualization of the PFA catheter in the Rhythmia system was done as reported elsewhere [13].
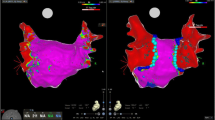
Pre- and post-ablation maps for left atrial linear ablations using a multipolar PFA catheter. Left atrial 3D-electroanatomical bipolar voltage maps for posterior wall isolation, mitral isthmus line ablation, and anterior line ablation using a multipolar PFA catheter. Pre-ablation maps were acquired during left atrial reentry tachycardia, post-ablation maps during sinus rhythm. The PFA catheter can be tracked by the 3D-EAM system and its position visualized in a simplified circular shape (lower row). Flower and basket shape cannot be distinguished. The scale for bipolar voltage maps is 0.05 mV to 0.5 mV. PFA = pulsed field ablation
Fluoroscopic guidance of catheter positioning during left atrial linear ablations using a multipolar PFA catheter. A1–A4 Ablation of a mitral isthmus line with the PFA catheter in either flower (A1 und A2) or basket configuration (A3 und A4). B1–B3 Roof line ablation with the catheter in flower configuration in different positions on the upper posterior wall. C1-C3 Catheter positioning for anterior line ablation. The flower configuration was used for the superior part of the anterior line (C1) and the basket configuration for ablation closer to the mitral annulus (C2 and C3). Lesion overlap was further verified by a 3D-EAM system (not shown here). PFA = pulsed field ablation; 3D-EAM = 3D-electroanatomical mapping
2.5 Roof line
With the guidewire in the superior PVs, an anchoring ablation was first performed with the catheter in flower configuration and the steerable sheath rotated towards the posterior wall. The remaining distance between the anchoring lesions in the LSPV and the RSPV was covered with overlapping ablations in flower configuration and with the guidewire retracted inside the catheter (Fig. 2). One ablation consisted of four applications in flower configuration, rotating the device 30°–40° after the first pair of applications for circumferential coverage. Another set of ablations in the same fashion on a more inferior aspect of the posterior wall was applied to complete LAPW isolation (Fig. 1). Complete LAPW isolation was confirmed by 3D-EAM showing no remaining signals on the posterior wall. In the case of remaining signals, PFA was repeated until LAPW isolation was complete.
2.6 Mitral isthmus line
The mitral isthmus line was ablated with the PFA catheter in flower or basket configuration, whichever yielded the best contact and coverage of the area of interest. Lesions were applied from the LIPV towards the mitral annulus (Figs. 1 and 2). If a bi-directional block according to standard criteria could not be achieved by PFA applications alone, the catheter was exchanged, and additional RF ablation was performed endocardially using 35 W, and epicardially in the great cardiac vein, using 20 W.
2.7 Anterior line
An anterior line was ablated connecting the RSPV to the anterior mitral annulus. An anchoring ablation was performed in the RSPV, with the device in flower configuration and the wire in the RSPV, rotating the sheath anteriorly. The same flower configuration was maintained for further applications on the superior aspect of the anterior wall, after retraction of the wire. For locations close to the anterior mitral annulus, ablations were performed with the catheter in basket configuration, ensuring tissue contact of one or two splines in the 3D-EAM (Figs. 1 and 2). For each ablation site, four applications were performed, rotating the catheter by 30°–40° after the first pair of applications. Bi-directional block across the anterior line according to standard criteria was assessed after completion of lesions. Additional PF or RF ablations were performed if no bi-directional block was achieved.
2.8 Statistical analysis
Continuous variables are presented as mean ± standard deviation or as median and interquartile range as appropriate. For statistical analyses, we used R 4.0.2 (R Core Team, Vienna, Austria).
3 Results
3.1 Patient characteristics
Patient and procedural characteristics are summarized in Table 1. The cohort consisted of 22 patients (59% male) with a median age of 70 years (IQR 59–75). Seventeen patients (77%) had prior LA ablation procedures, which included PVI in all and previous linear ablations in six cases (27%).
An overview of the mapping findings and the ablation lesion sets is presented in Tables 2 and 3. We used the 31-mm PFA device in 19 cases and the 35-mm PFA device in the remaining three cases. In twelve patients, all pulmonary veins were already isolated, and in five patients, the pulmonary veins were re-isolated by PFA. The remaining five patients had their first PVI by PFA. A total of 27 LA reentry tachycardia were observed and targeted by PFA. This included seven roof-dependent macro-reentries, 12 peri-mitral macro-reentries, one micro-reentry involving the posterior wall, and seven micro-reentry involving the anterior wall. We observed no acute procedural complications.
3.2 Roof-dependent macro-reentry and posterior, scar-related micro-reentry
Of the seven roof-dependent macro-reentries and one micro-reentry involving the posterior wall, five (63%) terminated during ablation of the roof line. For all patients receiving roof line ablation, LAPW isolation was also performed. Furthermore, a supplementary roof line and LAPW isolation was applied in 12 patients to avoid future occurrence of roof-dependent arrhythmia (Tables 2 and 3). No additional RF ablation was needed in any of the 20 patients to complete the roof line or to isolate the LAPW. No atrio-esophageal fistula occurred.
3.3 Peri-mitral macro-reentry
Among the twelve patients with ongoing peri-mitral macro-reentry, a standard mitral isthmus line was applied in six patients (50%) and an anterior line in the remaining six (50%; Tables 2 and 3). The latter all had an extensive anterior scar (Supplemental Fig. 1), and two showed anterior micro-reentry as well. Peri-mitral tachycardia terminated with PFA of the standard mitral isthmus line in four (67%) patients and during ablation of the anterior line in 6 (100%). Additional RF ablation to achieve bidirectional block of the mitral isthmus line and anterior line was needed in three (50%) and two (40%) patients respectively (35 W for endocardial ablation, 20 W for epicardial ablation in the great cardiac vein). In patients requiring additional RF ablation for the completion of the mitral isthmus line, RF ablation was performed within the great cardiac vein for all three cases and endocardially in one case. Bidirectional block of the standard mitral isthmus line and the anterior line was finally and acutely achieved in five (83%) and six (100%) patients with peri-mitral macro-reentry tachycardia, respectively.
In one patient, PFA of the anterior line resulted in transient, complete AV block, which led us to switch to RF ablation. However, transient, complete AV block also occurred with RF ablation and was finally attributed to a vagal reaction during ablation. After the application of 0.5 mg atropine, RF ablation at the same site was possible without the occurrence of complete AV block.
3.4 Anterior, scar-related micro-reentry
Of the seven anterior micro-reentry tachycardias, 6 (86%) terminated during PFA of the area of origin within an anterior scar region (Fig. 3). Two patients with anterior micro-reentries also had peri-mitral macro-reentry (see above). In five patients, the anterior line was applied using PFA exclusively. In the remaining two patients, additional RF ablation was necessary to achieve bidirectional block. One of those had an LAA occluder (Amplatzer Amulet, 31 mm), which prevented the delivery of PFA energy due to electrical interference with the catheter. Bidirectional block across the anterior line was not achieved in one patient (14%).
Anterior scar related reentry tachycardia. Bipolar voltage map (A) and local activation map (B) of a representative scar-related reentry tachycardia on the anterior wall of the left atrium. After ablation of an anterior line, the voltage map (C) displays a large, homogenous line of low amplitude. Activation map during pacing from the left lateral wall shows a blocked anterior line (D, white line). The scale for bipolar voltage maps is 0.05 mV to 0.5 mV
4 Discussion
In this cohort of 22 patients, we report our initial experience using a commercially available PFA catheter for the treatment of LA reentry tachycardia. Of the 39 lines attempted, a roof line and LAPW isolation was the target in 20 patients, an anterior line in 13, and a standard mitral isthmus line in 6. Bidirectional block could be achieved across 37 (95%) of the targeted lines, with supplementary RF ablation in 5 (14%) cases.
Due to the combination of procedural efficacy, procedural safety, and lesion durability, the non-thermal PFA technology has the potential to become the preferred technology for atrial fibrillation ablation. Most of the PFA work to date has focused on PVI [8, 12, 14, 15]. Accordingly, the design of the currently available PFA catheter has been optimized for PVI [8, 10, 14]. The advantages of PFA however make the technology also attractive for use outside the PVs [13, 16]. The use of the currently approved PFA catheter for linear ablation in patients with LA reentry tachycardia has been reported in case reports but was not systematically studied [17].
4.1 Roof line and left atrial posterior wall isolation
The multipolar PFA catheter is particularly well suited for the application of a roof line and for LAPW isolation. In comparison, ablation of a roof line or LAPW isolation by RF ablation is challenging. In a meta-analysis, the success rate for LAPW isolation with RF ablation was 71% (95% CI, 46–92%) [18]. Pak et al. used 3D-EAM guided touch-up ablations on local signals within the posterior wall in addition to a roof and posterior inferior line and reached a high success rate of acute, complete LAPW isolation in 94% of cases [19]. Those extended RF ablation strategies are comparable with LAPW isolation using PFA in terms of ablation area. However, PFA might have some distinct advantages over RF ablation: First, with RF, extensive ablation of the posterior wall must be applied cautiously to avoid esophageal damage. In that regard, the tissue-selective properties of PFA should reduce the risk of esophageal damage even with large-area ablation [20,21,22,23]. Indeed, two studies found no esophageal lesions on post-procedural esophagogastro-duodenoscopy after LAPW isolation using the same PFA system [13, 14]. Second, Pambrun et al. showed the importance of epicardial bypass in cases with residual gaps in the roof line [5]. By covering the entire posterior wall, the PFA approach also covers potential epicardial insertions into the LAPW.
In a pre-market trial, the same PFA device that was used in our study was successful in LAPW isolation in 25/25 patients [13, 14]. With a different PFA catheter platform allowing for focal lesions, application of a roof line using PFA was effective in 22/22 patients [24]. In aggregation, this means that the lesion depth of PFA at the roof and the posterior wall seems sufficient to also target epicardial bundles.
4.2 Standard mitral isthmus line
Ablation of a standard mitral isthmus line with RF ablation is successful in achieving bidirectional block in only 64% of patients [1]. The mitral isthmus is crossed by both the left circumflex coronary artery and the great coronary vein on the mitral side and by the vein of Marshal on the pulmonary venous side. These vessels are surrounded by myofibres, providing an epicardial, electrical bypass across linear lesions that are applied on the endocardial side [1]. Furthermore, the cooling effect of the blood flow of these vessels protects the tissue surrounding the veins from heating by RF ablation. To overcome these limitations, ethanol ablation of the vein of Marshal has proven very effective, although additional RF ablation on the mitral side of the mitral isthmus line or within the great cardiac vein is still necessary for a majority of patients [4, 7].
Using PFA, we were able to achieve acute bidirectional block across the mitral isthmus line in 83% of cases, including three cases in which additional RF ablation within the great cardiac vein was needed. Since the successful ablation of standard mitral isthmus line might still request additional ablation from within the great cardiac vein, a focal PFA catheter may be better suited. In a pre-clinical study, a different PFA system was used to deliver a combination of pulsed field and RF lesions from a single lattice-tip catheter both endo- and epicardially and achieved acute mitral isthmus block in 13/14 patients [24].
It remains to be demonstrated, whether the necessary lesion depth for durable block of the standard mitral isthmus line can be achieved by PFA. Gunawardene et al. reported successful mitral isthmus ablation using PFA in two patients without the need for additional RFA. However, in one case, coronary artery spasm occurred after eight PFA applications and resolved upon administration of nitroglycerin [17]. Although coronary arteries did not display particular susceptibility to PFA in animal models[23], more recent evidence showed that PFA routinely provokes coronary spasms when energy is delivered adjacent to coronary arteries [25]. In view of this new data, we currently avoid PFA in close proximity to large coronary arteries.
In our study, for the ablation of the mitral isthmus line, PFA lesions were applied in both the flower and basket configuration. The flower configuration can be used to target a larger area. The basket configuration might be used to optimize tissue contact of some splines of the PFA catheter at sites with residual gaps identified by 3D-EAM subsequent to PFA applications in the flower configuration.
4.3 Anterior line
Increased tissue thickness and epicardial connections may be obstacles to the application of an anterior line from the RSPV to the anterior mitral annulus. Ablation of an anterior line usually is not the first choice for the treatment of peri-mitral macro-reentry. However, this line might be the preferred approach in the presence of an extensive scar on the anterior wall, forming the substrate for both peri-mitral macro-reentry and anterior micro-reentry tachycardia. Using RF ablation, a report on a large cohort of 398 patients observed an acute success rate for block across an anterior line of 64% [3]. In our experience, the specific, PV-focused over-the-wire design of the multipolar PFA catheter seems suboptimal for ablation of an anterior line. The superior part of the line close to the RSPV was best attainable with the catheter in the flower configuration. A basket configuration was better suited to reach the mitral side of the anterior line. In particular, it is difficult to maintain good tissue contact on the anterior line, which is also a prerequisite for successful lesion application [26]. Despite extensive PFA, additional RF lesions were necessary in 2 cases (15%) to obtain bidirectional block and one anterior line could not be blocked, resulting in an acute success rate of 92%.
4.4 Integration of the PFA catheter into 3D-EAM systems
The multipolar PFA catheter can be used with simple fluoroscopic guidance and without the need for a 3D-EAM system. However, for planning and delivery of more complex lesion sets than PVI, real-time visualization using a 3D-EAM system allows for verification of appropriate catheter position before ablation. To identify the tachycardia mechanism of LA reentry tachycardia, 3D-EAM is used either way, and integration of the PFA catheter is an obvious option. Despite the current lack of magnetic tracking, impedance-based visualization of the multipolar PFA catheter in two established mapping systems was remarkably accurate. Overlap between lesions as well as proximity to the LA tissue can easily be verified and the catheter position adjusted as needed (Fig. 1). In addition, the identification of electrodes and their corresponding signals may help to identify residual gaps.
4.5 General remarks
Pulsed-field ablation can result in local myocardial capture, thereby terminating the tachycardia by the mechanism of overdrive pacing. Therefore, termination by PFA should not be interpreted as proof of the tachycardia’s mechanism nor as proof for an effective lesion application. Extensive use of PFA for linear ablation and LAPW isolation may impair left atrial pump function. Nakatani et al. compared the effect of PFA with radiofrequency ablation on left atrial mechanical and structural characteristics after pulmonary vein isolation [27]. They found better left atrial reservoir and pump function late after PFA compared to radiofrequency ablation. However, whether this also holds true for PFA outside of the pulmonary veins will need to be addressed by future studies.
4.6 Limitations
Potential limitations of the presented study merit consideration. First, this is a small cohort and represents a single-center experience at an early stage of the implementation of a new technology. Therefore, conclusions on the efficacy and safety of linear PFA should be drawn cautiously. Our results need external validation, ideally by the contribution of multiple centers. Second, the PFA system utilized in this study is labeled and designed for PVI. Dedicated PFA catheters for linear ablation have been developed, are undergoing pre-clinical testing, and will further improve results. Third, we report the acute results to achieve block across ablation lines. Despite favorable evidence accumulating on the durability of PFA lesions in PVI, the durability of linear PFA lesions is currently not known. Fourth, our experience is limited to a specific PFA system. Our results cannot be applied to other PFA platforms as they incorporate different ablation parameters and catheter designs.
5 Conclusion
Ablation of a roof line and LAPW isolation is feasible, effective, and safe with this multipolar PFA catheter. However, the catheter is less suited for ablation of the mitral isthmus and the anterior line, for which complementary RF ablation may be needed to achieve bidirectional block. A focal pulsed-field ablation catheter may be more effective for ablation of these lines.
Data availability
The authors had full access to and take full responsibility for the integrity of the data.
References
Yokokawa M, Sundaram B, Garg A, Stojanovska J, Oral H, Morady F, Chugh A. Impact of mitral isthmus anatomy on the likelihood of achieving linear block in patients undergoing catheter ablation of persistent atrial fibrillation. Heart Rhythm. 2011;8:1404–10. https://doi.org/10.1016/j.hrthm.2011.04.030.
Mujović N, Marinković M, Marković N, Stanković G, Lip GYH, Blomstrom-Lundqvist C, Bunch TJ, Potpara TS. Persistency of left atrial linear lesions after radiofrequency catheter ablation for atrial fibrillation: data from an invasive follow-up electrophysiology study. J Cardiovasc Electrophysiol. 2017;28:1403–14. https://doi.org/10.1111/jce.13322.
Kim T-H, Park J, Uhm J, Kim J, Joung B, Lee M, Pak H. Challenging achievement of bidirectional block after linear ablation affects the rhythm outcome in patients with persistent atrial fibrillation. J Am Heart Assoc. 2016;5:e003894. https://doi.org/10.1161/JAHA.116.003894.
Kamakura T, Derval N, Duchateau J, Denis A, Nakashima T, Takagi T, Ramirez FD, André C, Krisai P, Nakatani Y, et al. Vein of Marshall ethanol infusion: feasibility, pitfalls, and complications in over 700 patients. Circ Arrhythm Electrophysiol. 2021;14:e010001. https://doi.org/10.1161/CIRCEP.121.010001.
Pambrun T, Duchateau J, Delgove A, Denis A, Constantin M, Ramirez FD, Chauvel R, Tixier R, Welte N, André C, et al. Epicardial course of the septopulmonary bundle: anatomical considerations and clinical implications for roof line completion. Heart Rhythm. 2021;18:349–57. https://doi.org/10.1016/J.HRTHM.2020.11.008.
Buch E, Shivkumar K. Epicardial catheter ablation of atrial fibrillation. Minerva Med. 2009;100:151–7. https://doi.org/10.1161/CIRCEP.117.005748.
Lam A, Küffer T, Hunziker L, Nozica N, Asatryan B, Franzeck F, Madaffari A, Haeberlin A, Mühl A, Servatius H, et al. Efficacy and safety of ethanol infusion into the vein of Marshall for mitral isthmus ablation. J Cardiovasc Electrophysiol. 2021;32:1610–9. https://doi.org/10.1111/jce.15064.
Reddy VY, Neuzil P, Koruth JS, Petru J, Funosako M, Cochet H, Sediva L, Chovanec M, Dukkipati SR, Jais P. Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol. 2019;74:315–26. https://doi.org/10.1016/j.jacc.2019.04.021.
Koruth JS, Kuroki K, Kawamura I, Stoffregen WC, Dukkipati SR, Neuzil P, Reddy VY. Focal pulsed field ablation for pulmonary vein isolation and linear atrial lesions: a preclinical assessment of safety and durability. Circ Arrhythm Electrophysiol. 2020;13:e008716. https://doi.org/10.1161/CIRCEP.120.008716.
Kueffer T, Baldinger SH, Servatius H, Madaffari A, Seiler J, Mühl A, Franzeck F, Thalmann G, Asatryan B, Haeberlin A, et al. Validation of a multipolar pulsed-field ablation catheter for endpoint assessment in pulmonary vein isolation procedures. Europace. 2022;24(8):1248–55. https://doi.org/10.1093/europace/euac044 (In press).
Servatius H, Küffer T, Baldinger SH, Asatryan B, Seiler J, Tanner H, Novak J, Lam A, Noti F, Haeberlin A, et al. Dexmedetomidine versus propofol for operator-directed nurse-administered procedural sedation during catheter ablation of atrial fibrillation: A randomized controlled study. Heart Rhythm. 2022;19:691–700. https://doi.org/10.1016/j.hrthm.2021.12.028.
Reddy VY, Koruth J, Jais P, Petru J, Timko F, Skalsky I, Hebeler R, Labrousse L, Barandon L, Kralovec S, et al. Ablation of atrial fibrillation with pulsed electric fields: an ultra-rapid, tissue-selective modality for cardiac ablation. J Am Coll Cardiol EP. 2018;4:987–95. https://doi.org/10.1016/j.jacep.2018.04.005.
Gunawardene MA, Schaeffer BN, Jularic M, Eickholt C, Maurer T, Akbulak R, Flindt M, Anwar O, Pape UF, Maasberg S, et al. Pulsed-field ablation combined with ultrahigh-density mapping in patients undergoing catheter ablation for atrial fibrillation: Practical and electrophysiological considerations. J Cardiovasc Electrophysiol. 2022;33:345–56. https://doi.org/10.1111/jce.15349.
Reddy VY, Anic A, Koruth J, Petru J, Funasako M, Minami K, Breskovic T, Sikiric I, Dukkipati SR, Kawamura I, et al. Pulsed field ablation in patients with persistent atrial fibrillation. J Am Coll Cardiol. 2020;76:1068–80. https://doi.org/10.1016/j.jacc.2020.07.007.
Reddy VY, Dukkipati SR, Neuzil P, Anic A, Petru J, Funasako M, Cochet H, Minami K, Breskovic T, Sikiric I, et al. Pulsed field ablation of paroxysmal atrial fibrillation: 1-year outcomes of IMPULSE, PEFCAT, and PEFCAT II. J Am Coll Cardiol EP. 2021;7:614–27. https://doi.org/10.1016/j.jacep.2021.02.014.
Urbanek L, Chen S, Bordignon S et al. First pulse field ablation of an incessant atrial tachycardia from the right atrial appendage. J Interv Card Electrophysiol. (2022). https://doi.org/10.1007/s10840-022-01345-3.
Gunawardene MA, Schaeffer BN, Jularic M, Eickholt C, Maurer T, Akbulak RÖ, Flindt M, Anwar O, Hartmann J, Willems S. Coronary spasm during pulsed field ablation of the mitral isthmus line. J Am Coll Cardiol EP. 2021;7:1618–20. https://doi.org/10.1016/j.jacep.2021.08.016.
Thiyagarajah A, Kadhim K, Lau DH, Emami M, Linz D, Khokhar K, Munawar DA, Mishima R, Malik V, O’shea C, et al. Feasibility, safety, and efficacy of posterior wall isolation during atrial fibrillation ablation: a systematic review and meta-analysis. Circ Arrhythmia Electrophysiol. 2019;12:1–11. https://doi.org/10.1161/CIRCEP.118.007005.
Pak HN, Park J, Park JW, Yang SY, Yu HT, Kim TH, Uhm JS, Choi JI, Joung B, Lee MH, et al. Electrical posterior box isolation in persistent atrial fibrillation changed to paroxysmal atrial fibrillation. Circ Arrhythmia Electrophysiol. 2020;13:938–47. https://doi.org/10.1161/CIRCEP.120.008531.
Cochet H, Nakatani Y, Sridi-Cheniti S, Cheniti G, Ramirez FD, Nakashima T, Eggert C, Schneider C, Viswanathan R, Derval N, et al. Pulsed field ablation selectively spares the oesophagus during pulmonary vein isolation for atrial fibrillation. Europace. 2021;23:1391–9. https://doi.org/10.1093/europace/euab090.
Maor E, Sugrue A, Witt C, Vaidya VR, DeSimone CV, Asirvatham SJ, Kapa S. Pulsed electric fields for cardiac ablation and beyond: a state-of-the-art review. Heart Rhythm. 2019;16:1112–20. https://doi.org/10.1016/j.hrthm.2019.01.012.
Sugrue A, Vaidya V, Witt C, DeSimone CV, Yasin O, Maor E, Killu AM, Kapa S, McLeod CJ, Miklavčič D, et al. Irreversible electroporation for catheter-based cardiac ablation: a systematic review of the preclinical experience. J Interv Card Electrophysiol. 2019;55:251–65. https://doi.org/10.1007/s10840-019-00574-3.
Koruth J, Kuroki K, Iwasawa J, Enomoto Y, Viswanathan R, Brose R, Buck ED, Speltz M, Dukkipati SR, Reddy VY. Preclinical evaluation of pulsed field ablation: electrophysiological and histological assessment of thoracic vein isolation. Circ Arrhythmia Electrophysiol. 2019;12:1–9. https://doi.org/10.1161/CIRCEP.119.007781.
Reddy VY, Anter E, Rackauskas G, Peichl P, Koruth JS, Petru J, Funasako M, Minami K, Natale A, Jais P, et al. Lattice-tip focal ablation catheter that toggles between radiofrequency and pulsed field energy to treat atrial fibrillation: a first-in-human trial. Circ Arrhythm Electrophysiol. 2020;13:e008718. https://doi.org/10.1161/CIRCEP.120.008718.
Reddy VY, Petru J, Funasako M, Kopriva K, Hala P, Chovanec M, Janotka M, Kralovec S, Neuzil P. Coronary arterial spasm during pulsed field ablation to treat atrial fibrillation. Circulation. 2022;1–12. https://doi.org/10.1161/CIRCULATIONAHA.122.061497.
Meckes D, Emami M, Fong I, Lau DH, Sanders P. Pulsed-field ablation: computational modeling of electric fields for lesion depth analysis. Hear Rhythm O2. 2022;3:433–40. https://doi.org/10.1016/j.hroo.2022.05.009.
Nakatani Y, Sridi-Cheniti S, Cheniti G, Ramirez FD, Goujeau C, André C, Nakashima T, Eggert C, Schneider C, Viswanathan R, et al. Pulsed field ablation prevents chronic atrial fibrotic changes and restrictive mechanics after catheter ablation for atrial fibrillation. Europace. 2021;23:1767–76. https://doi.org/10.1093/europace/euab155.
Funding
Open access funding provided by University of Bern
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A. Haeberlin: research grants from the Swiss National Science Foundation, the Swiss Heart Foundation, the University of Bern, the University Hospital Bern, the Velux Foundation, the Hasler Foundation, the Swiss Heart Rhythm Foundation, and the Novartis Research Foundation. He is Co-founder and CEO of Act-Inno, a cardiovascular device testing company. He has received travel fees/educational grants from Medtronic, Philips/Spectranetics and Cairdac without impact on his personal remuneration. L. Roten: speaker honoraria from Abbott/SJM and consulting honoraria from Medtronic. T. Reichlin: research grants from the Swiss National Science Foundation, the Swiss Heart Foundation and the sitem-insel support fund. Speaker/consulting honoraria or travel support from Abbott/SJM, Bayer, Biosense-Webster, Biotronik, Boston-Scientific, Daiichi Sankyo, Medtronic, and Pfizer-BMS. Support for his institution’s fellowship program from Abbott/SJM, Biosense-Webster, Biotronik, Boston-Scientific, and Medtronic. F. Noti: Medtronic, Abbott (travel fees, speaker fees, educational grant); Boston Scientific, Philips Spectranetics (travel fees, educational grant); Biotronik (Institutional grant all for work outside the submitted study). A. Mühl: owns stock from Boston Scientific. J. Seiler: the spouse of Dr. Seiler is an employee and stock owner of Boston Scientific. All other authors report no conflicts of interest related to this paper.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kueffer, T., Seiler, J., Madaffari, A. et al. Pulsed-field ablation for the treatment of left atrial reentry tachycardia. J Interv Card Electrophysiol 66, 1431–1440 (2023). https://doi.org/10.1007/s10840-022-01436-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-022-01436-1