Abstract
Background
Pilot clinical studies suggest that very high power–very short duration (vHPvSD, 90 W/4 s, 360 J energy) is a feasible and safe technique for ablation of atrial fibrillation (AF), compared with standard applications using moderate power–moderate duration (30 W/30 s, 900 J energy). However, it is unclear whether alternate power and duration settings for the delivery of the same total energy would result in similar lesion formation. This study compares temperature dynamics and lesion size at different power-duration settings for the delivery of equivalent total energy (360 J).
Methods
An in silico model of radiofrequency (RF) ablation was created using the Arrhenius function to estimate lesion size under different power-duration settings with energy balanced at 360 J: 30 W/12 s (MPSD), 50 W/7.2 s (HPSD), and 90 W/4 s (vHPvSD). Three catheter orientations were considered: parallel, 45°, and perpendicular.
Results
In homogenous tissue, vHPvSD and HPSD produced similar size lesions independent of catheter orientation, both of which were slightly larger than MPSD (lesion size 0.1 mm deeper, ~ 0.7 mm wider, and ~ 25 mm3 larger volume). When considering heterogeneous tissue, these differences were smaller. Tissue reached higher absolute temperature with vHPvSD and HPSD (5–8 °C higher), which might increase risk of collateral tissue injury or steam pops.
Conclusion
Ablation for AF using MPSD or HPSD may be a feasible alternative to vHPvSD ablation given similar size lesions with similar total energy delivery (360 J). Lower absolute tissue temperature and slower heating may reduce risk of collateral tissue injury and steam pops associated with vHPvSD and longer applications using moderate power.







Similar content being viewed by others
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Halbfass P, Wielandts JY, Knecht S, le Polain De Waroux JB, Tavernier R, de Wilde V … Deneke T. Safety of very high-power short-duration radiofrequency ablation for pulmonary vein isolation: a two-centre report with emphasis on silent oesophageal injury. EP Europace. 2022;24(3):400–5. https://doi.org/10.1093/EUROPACE/EUAB261.
Stabile G, Schillaci V, Strisciuglio T, Arestia A, Agresta A, Shopova G … Solimene F. In vivo biophysical characterization of very high power, short duration, temperature-controlled lesions. Pac Clin Electrophysiol PACE. 2021;44(10):1717–23. https://doi.org/10.1111/PACE.14358.
Richard Tilz R, Sano M, Vogler J, Fink T, Saraei R, Sciacca V … Heeger CH. Very high-power short-duration temperature-controlled ablation versus conventional power-controlled ablation for pulmonary vein isolation: the fast and furious - AF study. Int J Cardiol Heart Vasc. 2021;35:100847. https://doi.org/10.1016/J.IJCHA.2021.100847.
Reddy VY, Grimaldi M, de Potter T, Vijgen JM, Bulava A, Duytschaever MF … Pürerfellner H. Pulmonary vein isolation with very high power, short duration, temperature-controlled lesions: the QDOT-FAST trial. JACC Clin Electrophysiol. 2019;5(7):778–86. https://doi.org/10.1016/J.JACEP.2019.04.009.
Nakagawa H, Ikeda A, Sharma T, Govari A, Ashton J, Maffre J … Jackman WM. Comparison of in vivo tissue temperature profile and lesion geometry for radiofrequency ablation with high power-short duration and moderate power-moderate duration effects of thermal latency and contact force on lesion formation. Circ Arrhythmia Electrophysiol. 2021;14:605–17. https://doi.org/10.1161/CIRCEP.121.009899.
Pérez JJ, González-Suárez A, Maher T, Nakagawa H, d’Avila A, Berjano E. Relationship between luminal esophageal temperature and volume of esophageal injury during RF ablation: in silico study comparing low power-moderate duration vs. high power-short duration. J Cardiovasc Electrophysiol. 2022;33(2):220–30. https://doi.org/10.1111/JCE.15311.
González-Suárez A, Pérez JJ, Irastorza RM, D’Avila A, Berjano E. Computer modeling of radiofrequency cardiac ablation: 30 years of bioengineering research. Comput Methods Programs Biomed. 2022;214:106546. https://doi.org/10.1016/J.CMPB.2021.106546.
Reporting of computational modeling studies in medical device submissions. Guidance for Industry and Food and Drug Administration staff. 2016. Retrieved from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/reporting-computational-modeling-studies-medical-device-submissions. Accessed 1 May 2022
Irastorza RM, Gonzalez-Suarez A, Pérez JJ, Berjano E, Erez JJP, Nacional A … Varela A. Differences in applied electrical power between full thorax models and limited-domain models for RF cardiac ablation. Int J Hyperth. 2020;37(1):677–87. https://doi.org/10.1080/02656736.2020.1777330.
Irastorza RM, d’Avila A, Berjano E. Thermal latency adds to lesion depth after application of high-power short-duration radiofrequency energy: results of a computer-modeling study. J Cardiovasc Electrophysiol. 2018;29(2):322–7. https://doi.org/10.1111/JCE.13363.
Hasgall PA, di Gennaro F, Baumgartner C. IT’IS database for thermal and electromagnetic parameters of biological tissues. Version 4.0. 2018. https://doi.org/10.13099/VIP21000-04-0
Pérez JJ, Ewertowska E, Berjano E. Computer modeling for radiofrequency bipolar ablation inside ducts and vessels: relation between pullback speed and impedance progress. Lasers Surg Med. 2020;52(9):897–906. https://doi.org/10.1002/lsm.23230.
Mitchell HH, Hamilton TS, Steggerda FR, Bean HW. The chemical composition of the adult human body and its bearing on the biochemistry of growth. J Biol Chem. 1945;158(3):625–37. https://doi.org/10.1016/s0021-9258(19)51339-4.
Organ LW. Electrophysiologic principles of radiofrequency lesion making. Appl Neurophysiol. 1976;32(2):69–76. https://doi.org/10.1159/000102478.
González-Suárez A, Pérez JJ, Berjano E. Should fluid dynamics be included in computer models of RF cardiac ablation by irrigated-tip electrodes? BioMed Eng Online 2018;17(1). https://doi.org/10.1186/s12938-018-0475-7
González-Suárez A, Berjano E, Guerra JM, Gerardo-Giorda L. Computational modeling of open-irrigated electrodes for radiofrequency cardiac ablation including blood motion-saline flow interaction. PLoS ONE. 2016;11(3):e0150356. https://doi.org/10.1371/journal.pone.0150356.
Pérez JJ, Nadal E, Berjano E, González-Suárez A. Computer modeling of radiofrequency cardiac ablation including heartbeat-induced electrode displacement. Comput Biol Med. 2022;144:105346. https://doi.org/10.1016/J.COMPBIOMED.2022.105346.
Masnok K, Watanabe N. Relationship of catheter contact angle and contact force with contact area on the surface of heart muscle tissue in cardiac catheter ablation. Cardiovasc Eng Technol. 2021;12(4):407–17. https://doi.org/10.1007/S13239-021-00529-8/FIGURES/7.
Masnok K, Watanabe N. Catheter contact area strongly correlates with lesion area in radiofrequency cardiac ablation: an ex vivo porcine heart study. J Interv Card Electrophysiol. 2022;63(3):561. https://doi.org/10.1007/S10840-021-01054-3.
de Potter T, Grimaldi M, Jensen H K, Kautzner J, Neuzil P, Vijgen J … Reddy VY. Temperature-controlled catheter ablation for paroxysmal atrial fibrillation: the QDOT-MICRO workflow study corresponding author. n.d. Retrieved from: www.jafib.com. Accessed 1 May 2022
Pearce JA. Comparative analysis of mathematical models of cell death and thermal damage processes. Int J Hyperth. 2013;29(4):262–80. https://doi.org/10.3109/02656736.2013.786140.
Haines DE. Letter by haines regarding article, “direct measurement of the lethal isotherm for radiofrequency ablation of myocardial tissue.” Circ Arrhythm Electrophysiol 2011;4(5). https://doi.org/10.1161/CIRCEP.111.965459
Funding
This study has been supported by the Spanish Ministerio de Ciencia, Innovación y Universidades/Agencia Estatal de Investigación IMCIN/AEI/10.13039/501100011033 (Grant RTI2018-094357-B-C21).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1
Appendix 1
1.1 Material properties
1.2 Boundary conditions
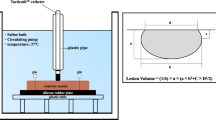
Each model (half of the entire domain, see Fig. 1) was made of ~ 214,000 tetrahedral elements and ~ 300,000 nodes. The outer dimensions (4 cm around the ablation electrode), mesh size (minimum of 118 μm around the electrode and maximum of 12 mm in the periphery), and the time step (ranging from 20 to 100 ms) were verified by means of convergence tests. The model solved a coupled electric-thermal problem numerically using the finite element method with ANSYS software (ANSYS, Canonsburg, PA, USA). Laplace’s equation and bioheat equation were used to solve the electrical and thermal problem, respectively. Details of these equations and boundary conditions are described in detail elsewhere [7]. The dispersive electrode was simulated using conditions of 0 V on all the outer surfaces except the symmetry plane. Electrode irrigation was modeled by fixing a value of 25 °C in the cylindrical zone of the electrode tip, and leaving the semispherical tip free, which mimics a multi-hole electrode since we are assuming that irrigation occupies almost the entire surface of the electrode [15]. The thermal problem was not solving in the blood, and thermal transfer coefficients for low blood flow condition (0.1 m/s) were used on the interfaces electrode-blood and myocardium-blood instead (3310 W/m2·K and 694 W/m2·K, respectively). A temperature of 37 °C was set at the outer contours of the tissue.
1.3 Thermal injury model
Lesion size (depth, maximum width, and volume) was computed using the thermal injury index Ω based on the Arrhenius model, which relates the number of undamaged cells C(0) present before the heating to the remaining number of undamaged cells at time t indicated by C(t) as follows:
It can be computed from the “thermal history” to which the tissue is subjected, specifically from the temperature T (in Kelvin) reached at each instant t (s) of the heating period:
where A is the frequency factor, Ea is the activation energy, and R the universal gas constant (8.3143 J/mol·K). The values of A and Ea are particular for each tissue type and analyzed process. In this study, the Ω = 1 isoline was considered to represent the thermal lesion contour. We considered A = 7.39·1039 s−1 and Ea = 2.577·105 J/mol, since these values were checked in a previous study[10] in order to provide a Ω = 1 isoline more or less coincident with the 72 °C isotherm for 5 s heating and more or less coincident with the 55 °C isotherm after 60 s of heating [21, 22]. To sum up, lesion size was volumetrically calculated by considering all the elements of the mesh in which Ω ≥ 1.
Rights and permissions
About this article
Cite this article
Pérez, J.J., D’Angelo, R., González-Suárez, A. et al. Low-energy (360 J) radiofrequency catheter ablation using moderate power − short duration: proof of concept based on in silico modeling. J Interv Card Electrophysiol 66, 1085–1093 (2023). https://doi.org/10.1007/s10840-022-01292-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-022-01292-z