Abstract
Introduction
Cardiac resynchronization therapy (CRT) improves outcomes in sinus rhythm, but the data in atrial fibrillation (AF) is limited. Atrio-ventricular junctional ablation (AVJA) has been proposed as a remedy. The objective was to test if AVJA results in LV end-systolic volume (ESV) reduction ≥ 15% from baseline to 6 months.
Methods
The trial was a prospective multicenter randomized trial in 26 patients with permanent AF who were randomized 1:1 to CRT-D with or without AVJA.
Results
LVESV improved similarly by at least 15% in 5/10 (50%) in the CRT-D-only arm and in 6/12 (50%) in the AVJA + CRT-D arm (OR = 1.00 [0.14, 7.21], p = 1.00). In the CRT-D-only arm, the median 6-month improvement in LVEF was 9.2%, not different from the AVJA + CRT-D arm, 8.2%. When both groups were combined, a significant increase in LVEF was observed (25.4% at baseline vs 36.2% at 6 months, p = 0.002). NYHA class from baseline to 6 months for all patients combined improved 1 class in 15 of 24 (62.5%), whereas 9 remained in the same class and 0 degraded to a worse class.
Conclusion
In patients with permanent AF, reduced LVEF, and broad QRS who were eligible for CRT, there was insufficient evidence that AVJA improved echocardiographic or clinical outcomes; the results should be interpreted in light of a smaller than planned sample size. CRT, however, seemed to be effective in the combined study cohort overall, suggesting that CRT can be reasonably deployed in patients with AF.
Trial registration
ClinicalTrials.gov Identifier: NCT02946853.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cardiac resynchronization therapy (CRT) accomplished with biventricular (BiV) pacing is a demonstrably effective device intervention for patients with broad QRS and left ventricular (LV) systolic dysfunction [1, 2]. The clinical benefits of CRT include improved survival, functional status, and quality of life and significant reduction in hospitalizations. CRT results in reversal of adverse pathologic remodeling which results in increased left ventricular (LV) ejection fraction (EF) and reduced cardiac volumes. The recent heart failure (HF) treatment guidelines from Europe and the USA [1, 2] classify CRT as a class I indication for patients in sinus rhythm, with LBBB and LVEF ≤ 35%.
The seminal clinical trials of CRT for advanced (classes III–IV) HF regularly restricted enrollment to patients in sinus rhythm. Hence, European and American guidelines [1, 2] did not strongly endorse the use of CRT in patients when sinus rhythm was not present. The rationale was that BiV pacing required the presence of organized atrial activity to ensure synchronized and consistent delivery of the ventricular pacing impulse. However, many patients with HF who are otherwise eligible for CRT are unable to maintain sinus rhythm and it is common in clinical practice to apply CRT to patients with persistent or permanent atrial fibrillation (AF). A recent report analyzing the NCDR US registry indicated that about 30% of CRT patients had AF or flutter [3].
The recent RAFT study of class II–III HF patients provided the largest sample of AF patients in a randomized clinical trial of CRT, but there was no difference in outcomes between the treatment and control groups [4]. However, patients in the CRT arm did not achieve substantial BiV pacing such that only 47% had > 90% pacing at 6 months of follow-up. These results would fall below the levels of BiV pacing which are believed to be required to generate a clinical benefit [5], despite the fact that patients were preselected to have well-controlled ventricular rates. To overcome the suboptimal response to CRT in AF patients, and to force obligate high level BiV pacing, AV junctional ablation (AVJA) has been employed in nonrandomized observational studies with results suggesting that benefit in these cohorts is seen only after AVJA [6,7,8,9,10].
In clinical practice, there is concern that using AVJA routinely will render many patients pacemaker-dependent, particularly in the absence of definitive data. In an effort to test the potential clinical benefit of routine AVJA and to determine feasibility for a large-scale randomized clinical trial, we designed the junctional AV ablation in patients with AF undergoing CRT (JAVA-CRT) randomized pilot program to test the hypothesis that the strategy of AVJA in patients with permanent AF who undergo CRT will result in improved LV remodeling as assessed by LV end-systolic volume (ESV) reduction ≥ 15% from baseline to 6 months.
1 Methods
This trial was supported by a grant from the National Heart, Lung, and Blood Institute (R34 HL133526) to the University of Rochester School of Medicine & Dentistry (Jonathan S. Steinberg, Principal Investigator). Each participating site’s institutional review board approved the study. Written informed consent was obtained from all patients.
JAVA-CRT trial was designed as an unblinded randomized controlled longitudinal trial in which patients were randomized 1:1 to undergo CRT-D with or without the addition of AVJA.
1.1 Study population
The enrollment criteria were deliberately strictly defined to identify patients with bonafide indications for CRT and continuous AF with rate controlled by medications. Excessively tachycardic patients despite medical therapy were deemed to be a priori AVJA candidates, and excessively bradycardic patients were deemed to be essentially pacemaker-dependent, and thus, both were not included in the trial. Specifically, patients eligible for trial enrollment included those with indications for initial implantation of CRT-D, with either ischemic or non-ischemic cardiomyopathy, LVEF ≤ 35%, and chronic LBBB (QRS ≥ 120 ms) or non-LBBB (QRS ≥ 150 ms) who had continuous AF > 6 months and in whom sinus rhythm was no longer pursued. Screening logs were not required to be maintained at enrolling sites (in large part because patients were evaluated for study participation after local referral for CRT from medical colleagues).
Initial inclusion criteria were:
-
Age > 21 years
-
Optimal pharmacologic therapy for heart failure and AF
-
LVEF ≤ 35%
-
NYHA class II-IV (ambulatory)
-
QRS ≥ 120 ms for LBBB and ≥ 150 ms for non-LBBB patients
-
Continuous AF > 6 months when no further efforts to restore sinus rhythm are feasible or pursued
-
Exclusion criteria were:
-
Ventricular rate > 110 bpm at rest despite maximal rate control medical therapy
-
Ventricular rate < 50 bpm at rest
-
Heart block/symptomatic bradycardia that necessitated permanent pacing
-
Acute coronary syndrome or coronary artery bypass surgery within 12 weeks
-
Severe aortic or mitral valvular heart disease
-
Prior AVJA
-
Any medical condition likely to limit survival to < 1 year
-
Patients with ACC/AHA stage D refractory class IV symptoms listed for transplant or requiring inotropic support
-
Contraindication to systematic anticoagulation
-
Renal failure requiring dialysis
-
AF due to a reversible cause
-
Pregnancy
-
Participation in other clinical trials that could affect the objectives of this study
-
History of non-compliance to medical therapy
-
Inability or unwillingness to provide informed consent
1.2 Trial procedures
At the discretion of the investigator, AVJA was performed immediately before or after implantation of a market-released CRT-D device. Cardiac AV conduction was monitored for at least 30 min following ablation to ensure the persistence of complete heart block with either ventricular asystole or a stable and regular escape rhythm. Ablation of the AV junction was targeted at the atrial side of the tricuspid valve annulus in an effort to create complete heart block at the level of the AV node. A deflectable ablation catheter was placed on the annulus where a large His-bundle electrogram was recordable, and then withdrawn until a large atrial electrogram and small His-bundle electrogram was visible. At this site, radiofrequency at 30–50 W was delivered for up to 60 s with the procedural endpoint of complete AV block. Provisions were made to perform AVJA at alternative sites if the standard approach was unsuccessful; however, the procedure had an anticipated success rate of 99%.
CRT-D devices were implanted in all patients, and for primary prevention patients, ICD programming followed MADIT-RIT arm B programming [11]. Specifically, a monitor-only zone for rates between 170 and 199 bpm was programmed, and active therapies were limited to rates of 200 bpm or greater. For secondary prevention patients, the ventricular tachycardia zone was programmed at a rate commensurate with the patient’s documented clinical arrhythmia.
All patients were maintained on rate-responsive BiV VVIR pacing throughout the study. In all patients, the pacemaker was programmed to a VVIR mode with a lower rate of 80 bpm for the first 4 weeks to mitigate the risk of polymorphic ventricular tachycardia for those patients undergoing AVJA. At 4 weeks, the pacemaker was programmed in a VVIR mode with a lower rate of 60 bpm and an upper sensor rate set at 80% of age-corrected maximum heart rate. Device algorithms designed to promote fusion and increased pacing during AF (e.g., ventricular sensed response, triggered pacing, and ventricular rate regularization) were programmed ON throughout the study.
Patients were required to be on optimal medical therapy including maximally tolerated doses of beta-blockers; angiotensin-converting enzyme inhibitors (ACEI); angiotensin receptor blockers (ARB) or ARB-neprilysin inhibitors (ARNI); aldosterone antagonists; and statins, digoxin, and aspirin, as recommended by current guidelines. Warfarin, or a direct oral anticoagulant, was used for stroke prevention. Beta-blockers and digoxin were used for rate control of ventricular response, as appropriate.
1.3 Trial endpoints
Patients were followed for 6 months, with follow-up contact scheduled at 3 months. Remote monitoring of the devices was used in the majority of patients to collect data regarding ICD therapies throughout their participation in the trial.
The primary endpoint of the study was LV reverse remodeling defined as a ≥ 15% reduction in LVESV from baseline to 6 months, a widely accepted endpoint used in many prior HF and CRT investigations. The primary endpoint of LV volume on echocardiogram, and all other echocardiographic variables, was analyzed in an echo core lab (under the direction of John Gorcsan, MD), blinded to patient treatment assignment.
Prespecified secondary endpoints included (a) change in LVEF from baseline to 6 months; (b) continuous LVESV and diastolic volumes, and the change in the latter from baseline to 6 months; (c) HF hospitalization; (d) all-cause mortality; (e) VT/VF arrhythmic events requiring ICD therapy; (f) inappropriate ICD therapy; (g) percentage BiV pacing; (h) quality of life scores; and (i) complications related to AVJA procedure. ICD interrogation data was interpreted by a core lab. HF hospitalizations and death data were ascertained from medical records and provided to a blinded endpoint adjudication committee.
Quality of life data was acquired at baseline and after 6 months of therapy using the Kansas City Cardiomyopathy Questionnaire (KCCQ) administered to the patient.
The trial was conducted under the auspices of a Data and Safety Monitoring Board that was responsible for monitoring the safety of the patients participating in this study and ensuring the ethical conduct of the trial. A main focus of DSMB activity was to monitor clinical adverse events that could be attributed to the ablation procedure. Data on clinical events, including procedural complications, HF hospitalizations, ventricular tachyarrhythmias requiring ICD therapy, and death were collected by the study and provided to the Board for review.
1.4 Statistical analysis
The primary analysis was a modified intention-to-treat (ITT) analysis based on Fisher’s exact test of the null hypothesis that the response rates were identical in both arms, conditional on the total number of responders, with the modification excluding subjects for whom 6-month outcomes were not obtained. The response rate for each arm was estimated by the empirical proportion of responders, and the treatment effect was summarized via the estimated odds ratio (OR) and associated 95% confidence interval (CI). Continuous secondary endpoints were compared across arms using Wilcoxon and t-tests and summarized via means, medians, and standard deviations. Counts and proportions were used to summarize the distributions of NYHA class, a discrete clinical endpoint. The paired Wilcoxon signed rank test was used to test 6-month changes from baseline for the following parameters: echocardiographic variables, KCCQ scores, and percent of pacing from CRT-D interrogations. All tests were two-sided.
1.5 Initial and revised sample size considerations; protocol amendments based on enrollment
Randomizing 40 subjects per arm (for a total of 80 subjects), and allowing for up to 10% drop-out due to withdrawal, death, or inadequate imaging, we expected at least n = 36 evaluable subjects per arm. This sample size provided 82–97% power to detect an absolute change of 35–45% in response rates, using a 0.05 level 2-sided Fisher exact test, assuming the actual response rate in the AVJA arm was 80–85%, as previously observed [6].
As enrollment proved challenging at all sites, subsequent study trial amendments reduced the requirement of confirmed AF duration to 3 months, allowed patients who had a planned upgrade from a prior implantable cardioverter-defibrillator (ICD) to CRT-D, and permitted enrollment after prior CRT within 12 months who had no observed response, or after failed AF ablation, all in an effort to increase the pool of eligible patients when enrollment proved challenging. In addition, the trial leadership, with agreement from DSMB, requested and received 2 consecutive 1-year trial extensions. Enrollment commenced in March 2017 and concluded in June 2020.
Given the observed enrollment, the revised eligibility criteria, and the objective in a pilot program to determine feasibility, the sample size was recalculated after 1 year of study activity. A total of 40 patients (20 per arm) provided 80% power to detect a change of 50% in response rates from 80% in the treatment arm versus 30% in the control arm, using a 2-sided 0.05 level Fisher exact test based on a total of 36 evaluable patients (18 per arm), assuming a 10% drop-out rate.
2 Results
2.1 Patients
Each group contained 13 patients who were well matched in key clinical characteristics and treatments (Table 1). In general, patients were older and in their 70 s, with about one-quarter female. Most had experienced NYHA class III HF due to non-ischemic cardiomyopathy and had elevated BNP despite treatment with the array of guideline-indicated medical therapy including both beta-blocker and ACEI, ARB, or ARNI in 77–85% of patients, respectively. AF history was prolonged over many years; and at entry, consistent treatment with rate control medications and anticoagulation was present. The most common ECG indication for CRT was LBBB. LVEF was significantly reduced with dilated LV and LA.
2.2 Procedures
All 26 patients underwent successful CRT implantation, including 23 at the time of randomization and 3 prior to randomization (1, 5, and 9 months, respectively). The LV lead positions for the new implantations were as follows: lateral (n = 11), posterolateral (n = 5), anterolateral (n = 4), posterior (n = 2), and anterior (n = 1). There were no device implant complications at or following the procedure; there were no lead or system revisions during follow-up.
The AVJA procedure was successfully accomplished in all 13 randomized patients leading to complete heart block, and all were successful at the superior tricuspid annulus target position. There were no complications related to the ablation procedure. The CRT procedure was performed on the same day in 8 patients, and within 1 day in 2 patients. The remaining 3 patients had CRT-D implants prior to randomization. During follow-up, no patient was observed to have resumption of AV conduction.
2.3 Study endpoints
2.3.1 Echocardiographic variables including primary endpoint
The 6-month echo was not completed in 3 patients in the CRT-D-only arm (1 due to patient death, and 2 to COVID-19 precautions) and in 1 patient in the AVJA + CRT-D arm (due to COVID-19 precautions). LVESV improved by at least 15% in 5/10 patients (50%) in the CRT-D-only arm and in 6/12 patients (50%) in the AVJA + CRT-D arm (OR = 1.00 [0.14, 7.21], p = 1.00).
Table 2 shows values of key echocardiographic parameters analyzed as continuous variables at baseline, at 6 months, and change between 6 months and baseline, with p values comparing changes by randomization arms. There was insufficient evidence of any difference between randomization arms in the change in LV ejection fraction, LV systolic and diastolic volumes, or LA diameter (p > 0.10 for all). LA volume was reduced in patients randomized to AVJA vs. CRT-D-only arm (p = 0.05).
Table 3 shows the results of further analyses of changes in echocardiographic variables from baseline to 6 months, for each randomized group separately, as well as for both groups combined (in a post hoc analysis to illustrate the overall effect of CRT on studied parameters). In the CRT-D-only arm, the median relative 6-month change in LVEF was 9.2% (p = 0.131), and in the AVJA + CRT-D arm, it was 8.2% (p = 0.012). Figure 1 presents data of individual patients at baseline and 6 months, sorted by baseline LVEF, with the vast majority of patients in each arm exhibiting an increase in LVEF. Of note, a small number of patients had LVEF > 35% at baseline, representing a discrepancy between screening EF at site vs that calculated by echo core lab. Figure 2 shows LVEF at baseline and at 6 months in patients from both arms combined: median at baseline = 25.4% vs. 36.2% at 6 months (p = 0.002). Table 3 also contains additional data items of LVESV, LVEDV, LA volume, and LV global longitudinal strain. LVESV, LA volume, and LV global longitudinal strain were improved at 6 months from baseline in the AVJA + CRT-D arm, but not in the CRT-D-only arm, although there was insufficient evidence of a difference between the arms. The median relative 6-month change in LVESV was − 10.5% (p = 0.492) in the CRT-D-only arm and − 23.0% (p = 0.05) in the AVJA + CRT-D arm. The median relative 6-month change in LA volume was 0.8% (p = 0.846) for CRT-D only and − 21.6% (p = 0.005) for AVJA + CRT-D.
2.3.2 Clinical outcomes including secondary endpoints
At 6 months, the AVJA + CRT-D group achieved a BiV pacing prevalence of 97.7 ± 3.6% vs only 77.1 ± 26.3% in the CRT-D-only group (p = 0.005). In the AVJA + CRT-D group, 91% of patients exceeded 90% biventricular pacing, whereas only 45% of the CRT-D group did so (p = 0.063). No patient crossed over from the CRT-D-only group to undergo AVJA.
One patient in the CRT-D only group died after hospitalization with HF and appropriate device shock for VT. In the AVJA + CRT-D group, no patient died or had device therapy for VT, and one patient was hospitalized with HF.
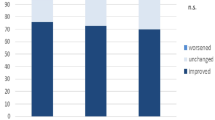
Figure 3 depicts the changes in NYHA class of HF from baseline to 6 months, by arm. In the CRT-D-only arm, 5 of 11 patients (45%) improved NYHA class vs. 10 of 13 (77%) in the CRT-D with AVJA arm (p = 0.206).
Both groups experienced improved KCCQ quality of life scores, notably the overall summary score but also most of the component scores (Table 4). Median overall summary score improved from 54.7 at baseline to 64.1 at 6 months in CRT-D-only patients (p = 0.083). In the CRT-D with AVJA, the median overall score improved from 65.9 to 89.6 (p = 0.002). There was insufficient evidence of any difference between arms.
2.3.3 Effect of CRT-D on studied parameters
The sections above indicate that both study groups generally responded to treatment assignment in the same directional manner. Herein we describe outcomes in the combined cohort of all study patients, irrespective to treatment assignment, in a non-prespecified post hoc analysis.
For all patients combined, the median relative 6-month change in LVESV was − 22.5% (p = 0.045) (Table 3). The median relative 6-month change in LVEF was 8.2% (p = 0.002) (Fig. 2). The median relative 6-month change in LA volume was − 13.6% (p = 0.06).
Among subjects with baseline LBBB (n = 17), the median relative 6-month change in LVESV was − 16% vs. 12% in non-LBBB subjects (n = 5) (p = 0.06). Among subjects with diagnosis of non-ischemic cardiomyopathy (n = 13), the median relative 6-month change in LVESV was − 19% vs 1% in ischemic cardiomyopathy subjects (n = 9) (p = 0.06). There was insufficient evidence of a difference in the 6-month relative change in LVESV for those above (> 111 ml) vs below (≤ 111 ml) the median baseline LVESV. Among patients with > 90% of BiV pacing, median 6-month change in LVESV was 13.4% vs 7.7% in those with ≤ 90% BiV pacing (p = 0.32).
The change in NYHA class of heart failure from baseline to 6 months for all patients combined is shown in Fig. 4. Overall, 15 of 24 patients for whom status was available demonstrated improvement of 1 NYHA class, 9 stayed in the same class, and 0 degraded to a worse class.
When analyzing the overall effect of CRT-D on KCCQ scores in both arms combined, most scores improved over the 6-month follow-up (bottom panel of Table 4), and the overall KCCQ summary score improved from a median of 63.3 at baseline to 83.9 at 6 months (p < 0.001).
3 Discussion
JAVA-CRT is the first randomized clinical trial to test the strategy of AVJA to improve the response to CRT in eligible patients with permanent AF. In large part due to small sample size, the study failed to establish the superiority of AVJA at the time of CRT implant versus CRT alone based on the absence of difference in the primary endpoint, the proportion of patients with improved LVESV of at least 15%. Because of statistical equivalency in the two study groups with AF, we also observed that both groups combined experienced benefit from CRT using a variety of relevant and meaningful clinical and echocardiographic endpoints. The study results were hampered by difficulty meeting the sample size targets, resulting in lower than planned power and precision.
All sites experienced challenges in meeting the enrollment targets despite the pre-launch documentation that a significant minority of patients who are referred for CRT have AF [3]. It was the collective opinion of the investigators that AF patients were not being referred to the same degree as in the past. We surmised that this drop-off was due to a perception that AF patients might not respond well to CRT, as documented in the most influential study of CRT in AF, RAFT [4]. Guidelines of heart failure management [1, 2] also have not awarded a clear class I indication for CRT in this subpopulation, further blunting referrals. In addition, there was emerging evidence that patients with AF, heart failure, and an ICD might have improved outcomes if catheter ablation were undertaken to directly suppress AF [12], a strategy that would siphon patients away from study participation. To counter the reduced enrollment, the Steering Committee liberalized the eligibility criteria to include patients with prior failed ablation, those with need for upgrade to CRT from an ICD, nonresponse to prior CRT, and a lesser confirmed duration of AF (although retaining requirement that no efforts be made to restore sinus rhythm). A revised calculation of sample size was undertaken. A battery of additional recruitment efforts was also made in collaboration with individual sites, as well as adding new US centers when it became impossible to include the planned European investigators (due to budget constraints). Further interfering with study enrollment and conduct was the outbreak of the COVID-19 pandemic in the USA. The ultimate result was a lower than planned sample size, power, and precision. Given the absolute and relative changes in LVESV observed in JAVA-CRT, to achieve 80–90% power, a study would require a total of 840–1120 patients to test the null hypothesis, and even larger if using hard endpoints such as HF hospitalization and death. Thus, we believe the possibility of conducting a large-scale trial of AF patients and CRT would be very challenging, if not unfeasible, in the current environment. Perusal of ClinicalTrials.gov reveals many trials of similar populations have been terminated as well.
Patients with AF have no atrial-ventricular (AV) synchrony so coordinated AV pacing with optimally timed AV intervals is impossible. Thus, BiV pacing delivery, and more importantly capture, cannot be reliably assured. Even when pacing is delivered, many ventricular complexes might be fused or pseudofused, making pacing capture percentages retrievable from the CRT device inaccurate and an overestimate of effective pacing capture [13]. The amount of BiV pacing is important as it is generally believed that near maximal effective and complete BiV capture is necessary to assure optimal CRT response [5].
The RAFT study of class II–III HF patients provided the largest sample of AF patients in a randomized clinical trial [4]. Enrollment was stratified based on the presence or absence of permanent atrial fibrillation. Of the 1798 patients in RAFT, 12.7% or 229 had permanent AF and 114 were randomized to CRT-D and 115 to ICD. The primary endpoint was heart failure hospitalization or death, and there was no difference between the 2 groups (HR = 0.96, p = 0.82) with survival curves that were superimposable. However, patients in the CRT-D arm did not achieve substantial BiV pacing: during the first 6 months after randomization, only 34% had > 95% pacing and only 47% had > 90% pacing. AVJA was rarely employed.
It had been postulated that therapeutic CRT was challenging in the setting of AF based on the twin necessity of achieving both quantitative and qualitative BiV pacing. One proposed solution was the addition of AVJA which would render the patient pacemaker-dependent, thus ensuring reliably and consistently delivered CRT without fusion/pseudofusion or intermittent rapid or irregular RR patterns. The initial results achieved with AVJA were encouraging, although not tested in a randomized trial. Gasparini et al. described the outcome of a large series of patients treated with CRT at 2 European centers [6], and only the patients with AF who had undergone AVJA demonstrated evidence of reverse remodeling and functional improvement. Furthermore, meta-analyses of published observational studies supported the value of AVJA [8, 9].
In the randomized and unblinded JAVA-CRT, we were unable to confirm the critical importance of AVJA for CRT in AF patients. Both patients with and without AVJA responded to CRT, although by some measures the response tended to be more pronounced in the AVJA arm (greater improvement in LVESV, LV global longitudinal strain, LA volume, NYHA class, and quality of life).
Although JAVA-CRT was underpowered, the comparative results between study arms suggest that a study with a very large sample size (see above) would be required to determine if there is a genuine and measurable benefit to AVJA in this setting, a trial that is likely not logistically feasible.
Because both patients with and without AVJA appeared to respond to CRT in JAVA-CRT, we combined groups to analyze the effects of the common therapeutic intervention, CRT, in patients with AF. We found a multifaceted apparent response including improved LVESV by reverse remodeling, increased LV systolic function and EF, and less heart failure functional impairment with lower NYHA class, and better quality of life by formal testing. Supportive of CRT benefit is the pattern of better responses in patients with LBBB vs non-LBBB, and in non-ischemic vs ischemic cardiomyopathy, typically observed in randomized trials. It should be noted that the overall group did not have a control group without CRT and confounding factors could have played a role in the study’s final results in comparison to baseline.
The response among control patients is also noteworthy because overall they did not achieve the high-level BiV pacing (in contra-distinction to the AVJA group) believed to be needed for CRT to be effective. The reasons are unclear but might suggest a lower threshold for necessary pacing prevalence in AF patients, especially if there is good rate control by medical therapy prior to device placement, as required in this trial.
These observations suggest that CRT is reasonable to employ in otherwise eligible patients who have permanent AF, and that AVJA is not obligatory, at least at time of implant. This is particularly important since avoiding AVJA avoids pacemaker dependence. Thus, AVJA can be held in reserve for nonresponders, although this concept was not formally tested in JAVA-CRT, using a procedure that was found to be consistently feasible and safe.
3.1 Limitations
The most important limitation of the study was a smaller than planned sample size, as discussed in detail above. In addition, the trial did not have a study arm without any intervention (either CRT or AVJA) so that “untreated” patients could be compared to each intervention group. The study was randomized, but the small sample size could also have produced imbalances in clinical characteristics between study groups. It should be noted that both treatment arms had rigorous medical therapy, including the introduction of ARNI therapy in a subset. Because patients in clinical trials typically have strict adherence to guideline-directed medical therapy, it may be difficult to confidently distinguish device-based treatment effects from benefits from pharmacological therapy. Hard clinical endpoints including hospitalization, death, and ICD events were too infrequent to make meaningful comparisons.
3.2 Conclusions
In patients with permanent AF, reduced LVEF and broad QRS who were eligible for CRT, there was insufficient evidence that AVJA improved echocardiographic or clinical outcomes; the results should be interpreted in light of a smaller than planned sample size. CRT, however, seemed to be effective in the combined study cohort overall, suggesting that CRT can be reasonably deployed in AF patients.
References
Voors AA, Anker SD, Bueno H, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2129–200.
Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guidelines for the management of heart failure. J Am Coll Cardiol. 2013;62:e147-239.
Echouffo-Tcheugui JB, Masoudi FA, Bao H, Curtis JP, Heidenreich PA, Fonarow GC. Body mass index and outcomes of cardiac resynchronization with implantable cardioverter-defibrillator therapy in older patients with heart failure. Eur J Heart Fail. 2019;21:1093–102.
Healy JS, Hohnloser SH, Exner DV, et al. Cardiac resynchronization therapy in patients with permanent atrial fibrillation; results from the Resynchronization for Ambulatory Heart Failure Trial (F-RAFT). Circ Heart Fail. 2012;5:566–70.
Hayes DL, Boehmer JP, Day JD, et al. Cardiac resynchronization therapy and the relationship of percent biventricular pacing to symptoms and survival. Heart Rhythm. 2011;8:1460–75.
Gasparini M, Auricchio A, Regoli F, et al. Four-year efficacy of cardiac resynchronization therapy on exercise tolerance and disease progression: the importance of performing atrioventricular junction ablation in patients with atrial fibrillation. J Am Coll Cardiol. 2006;48:734–43.
Gasparini M, Auricchio A, Metra M, et al. Long-term survival in patients undergoing cardiac resynchronization therapy: the importance of performing atrio-ventricular junction ablation in patients with permanent atrial fibrillation. Eur Heart J. 2008;29:1644–52.
Ganesan AN, Brooks AG, Roberts-Thomson KC, Lau DH, Kalman JM, Sanders P. Role of AV nodal ablation in cardiac resynchronization in patients with coexistent atrial fibrillation and heart failure. J Am Coll Cardiol. 2011;59:719–26.
Wilton SB, Leung AA, Ghali WA, Faris P, Exner DV. Outcomes of cardiac resynchronization therapy in patients with versus without atrial fibrillation: a systematic review and meta-analysis. Heart Rhythm. 2011;8:1088–94.
Dong K, Shen W-K, Powell BD, et al. Atrioventricular nodal ablation predicts survival benefit in patients with atrial fibrillation receiving cardiac resynchronization therapy. Heart Rhythm. 2010;7:1240–5.
Moss AJ, Schuger C, Beck CA, et al. Reduction in inappropriate therapy and mortality through ICD programming. N Eng J Med. 2012;367:2275–83.
Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–27.
Kamath GS, Cotiga D, Koneru JN, et al. The utility of 12-lead Holter monitoring in patients with permanent atrial fibrillation for the identification of nonresponders after cardiac resynchronization therapy. J Am Coll Cardiol. 2009;53:1050–5.
Funding
Funded by a grant from the National Heart, Lung, and Blood Institute (R34 HL133526) to the University of Rochester School of Medicine & Dentistry (Jonathan S. Steinberg, Principal Investigator).
Each participating site’s institutional review board approved the study. Written informed consent was obtained from all patients.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JSS (research support from NHLBI, AliveCor, Atricure, Allergan; consulting with Medtronic, Corfigo, Hillrom, Braveheart, National Cardiac, Cardioelectra; equity with AliveCor, Braveheart, National Cardiac); JG (research support from EBR Systems, V-Wave Ltd, AADi Pharmaceuticals); SKJ (research support from Medtronic, Boston Scientific and Abbott); WZ (research support from Boston Scientific, Biotronik, LivaNova; consulting with Medtronic, Abbott). The remaining authors have indicated that they have no relevant conflicts to report.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Data and safety monitoring board
Robert Goldstein, MD (chair), Mark Haigney, MD, Stuart Russell, MD, Thomas Louis, PhD, and Felicia Cohn, PhD.
Enrolling sites
University of Iowa, University of Pittsburgh, Huntington Memorial Hospital, Portland VA Medical Center, Saint Alphonsus Regional Medical Center, Multicare Institute for Research and Innovation, Northwell Hospital System, Kootenai Heart Clinics, Arkansas Cardiology, Lahey Clinic, University of Rochester Medical Center, Oregon Health & Science University, University of Pennsylvania, SUNY Buffalo, Drexel University College of Medicine, University of Massachusetts-Worcester, Inova Fairfax Hospital, William Beaumont Hospital, Abington Memorial Hospital, Emory Healthcare, and Catholic Medical Center.
Rights and permissions
About this article
Cite this article
Steinberg, J.S., Gorcsan, J., Mazur, A. et al. Junctional AV ablation in patients with atrial fibrillation undergoing cardiac resynchronization therapy (JAVA-CRT): results of a multicenter randomized clinical trial pilot program. J Interv Card Electrophysiol 64, 519–530 (2022). https://doi.org/10.1007/s10840-021-01116-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-021-01116-6