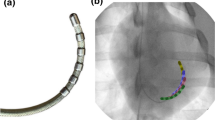
Abstract
Purpose
To demonstrate the feasibility of directional percutaneous epicardial ablation using a partially insulated catheter.
Methods
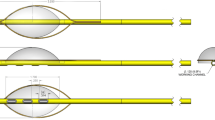
Partially insulated catheter prototypes were tested in 12 (6 canine, 6 porcine) animal studies in two centers. Prototypes had interspersed windows to enable visualization of epicardial structures with ultrasound. Epicardial unipolar ablation and ablation between two electrodes was performed according to protocol (5–60 W power, 0–60 mls/min irrigation, 78 s mean duration).
Results
Of 96 epicardial ablation attempts, unipolar ablation was delivered in 53.1%. Electrogram evidence of ablation, when analyzable, occurred in 75 of 79 (94.9%) therapies. Paired pre/post-ablation pacing threshold (N = 74) showed significant increase in pacing threshold post-ablation (0.9 to 2.6 mA, P < .0001). Arrhythmias occurred in 18 (18.8%) therapies (11 ventricular fibrillation, 7 ventricular tachycardia), mainly in pigs (72.2%). Coronary artery visualization was variably successful. No phrenic nerve injury was noted during or after ablation. Furthermore, there were minimal pericardial changes with ablation.
Conclusions
Epicardial ablation using a partially insulated catheter to confer epicardial directionality and protect the phrenic nerve seems feasible. Iterations with ultrasound windows may enable real-time epicardial surface visualization thus identifying coronary arteries at ablation sites. Further improvements, however, are necessary.






Similar content being viewed by others
Abbreviations
- ECG:
-
Electrocardiography
- EGM:
-
Electrogram
- ICE:
-
Intracardiac echocardiography
- IVUS:
-
Intravascular ultrasound
- RF:
-
Radiofrequency
- VF:
-
Ventricular fibrillation
- VT:
-
Ventricular tachycardia
References
Cano O, Hutchinson M, Lin D, Garcia F, Zado E, Bala R, et al. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol. 2009;54(9):799–808. https://doi.org/10.1016/j.jacc.2009.05.032.
Garcia FC, Bazan V, Zado ES, Ren JF, Marchlinski FE. Epicardial substrate and outcome with epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2009;120(5):366–75. https://doi.org/10.1161/CIRCULATIONAHA.108.834903.
Yamada T, McElderry HT, Allison JS, Kay GN. Focal atrial tachycardia originating from the epicardial left atrial appendage. Heart Rhythm. 2008;5(5):766–7. https://doi.org/10.1016/j.hrthm.2007.12.025.
Sacher F, Roberts-Thomson K, Maury P, Tedrow U, Nault I, Steven D, et al. Epicardial ventricular tachycardia ablation. A multicenter safety study. J Am Coll Cardiol. 2010;55(21):2366–72. https://doi.org/10.1016/j.jacc.2009.10.084.
Killu AM, Friedman PA, Mulpuru SK, Munger TM, Packer DL, Asirvatham SJ. Atypical complications encountered with epicardial electrophysiological procedures. Heart Rhythm. 2013;10(11):1613–21. https://doi.org/10.1016/j.hrthm.2013.08.019.
Starek Z, Lehar F, Jez J, Wolf J, Kulik T, Zbankova A, et al. Periprocedural 3D imaging of the left atrium and esophagus: comparison of different protocols of 3D rotational angiography of the left atrium and esophagus in a group of 547 consecutive patients undergoing catheter ablation of the complex atrial arrhythmias. Int J Cardiovasc Imaging. 2016;32:1011–9. https://doi.org/10.1007/s10554-016-0888-y.
Kumar S, Barbhaiya CR, Baldinger SH, Koplan BA, Maytin M, Epstein LM, et al. Epicardial phrenic nerve displacement during catheter ablation of atrial and ventricular arrhythmias: procedural experience and outcomes. Circ Arrhythm Electrophysiol. 2015;8(4):896–904. https://doi.org/10.1161/CIRCEP.115.002818.
Buch E, Vaseghi M, Cesario DA, Shivkumar K. A novel method for preventing phrenic nerve injury during catheter ablation. Heart Rhythm. 2007;4(1):95–8. https://doi.org/10.1016/j.hrthm.2006.09.019.
Bugge E, Nicholson IA, Thomas SP. Comparison of bipolar and unipolar radiofrequency ablation in an in vivo experimental model. Eur J Cardiothorac Surg. 2005;28(1):76–80; discussion −2. https://doi.org/10.1016/j.ejcts.2005.02.028.
van Driel VJ, Neven K, van Wessel H, Vink A, Doevendans PA, Wittkampf FH. Low vulnerability of the right phrenic nerve to electroporation ablation. Heart Rhythm. 2015;12(8):1838–44. https://doi.org/10.1016/j.hrthm.2015.05.012.
Neven K, van Driel V, van Wessel H, van Es R, du Pre B, Doevendans PA, et al. Safety and feasibility of closed chest epicardial catheter ablation using electroporation. Circ Arrhythm Electrophysiol. 2014;7(5):913–9. https://doi.org/10.1161/CIRCEP.114.001607.
Sosa E, Scanavacca M, d'Avila A, Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996;7(6):531–6.
Schwartzman D, Michele JJ, Trankiem CT, Ren JF. Electrogram-guided radiofrequency catheter ablation of atrial tissue comparison with thermometry-guide ablation: comparison with thermometry-guide ablation. J Interv Card Electrophysiol. 2001;5(3):253–66.
Sapp JL, Soejima K, Cooper JM, Epstein LM, Stevenson WG. Ablation lesion size correlates with pacing threshold: a physiological basis for use of pacing to assess ablation lesions. Pacing Clin Electrophysiol. 2004;27(7):933–7. https://doi.org/10.1111/j.1540-8159.2004.00561.x3.
Ring ME, Huang SK, Gorman G, Graham AR. Determinants of impedance rise during catheter ablation of bovine myocardium with radiofrequency energy. Pacing Clin Electrophysiol. 1989;12(9):1502–13.
Linhart M, Mollnau H, Bitzen A, Wurtz S, Schrickel JW, Andrie R, et al. In vitro comparison of platinum-iridium and gold tip electrodes: lesion depth in 4 mm, 8 mm, and irrigated-tip radiofrequency ablation catheters. Europace. 2009;11(5):565–70. https://doi.org/10.1093/europace/eup040.
Panescu D, Whayne JG, Fleischman SD, Mirotznik MS, Swanson DK, Webster JG. Three-dimensional finite element analysis of current density and temperature distributions during radio-frequency ablation. IEEE Trans Biomed Eng. 1995;42(9):879–90. https://doi.org/10.1109/10.412649.
Nguyen DT, Moss JD, Zheng L, Huang J, Barham W, Sauer WH. Effects of radiofrequency energy delivered through partially insulated metallic catheter tips on myocardial tissue heating and ablation lesion characteristics. Heart Rhythm. 2015;12(3):623–30. https://doi.org/10.1016/j.hrthm.2014.11.022.
d’Avila A, Houghtaling C, Gutierrez P, Vragovic O, Ruskin JN, Josephson ME, et al. Catheter ablation of ventricular epicardial tissue: a comparison of standard and cooled-tip radiofrequency energy. Circulation. 2004;109(19):2363–9. https://doi.org/10.1161/01.CIR.0000128039.87485.0B.
Suzuki Y, Yeung AC, Ikeno F. The representative porcine model for human cardiovascular disease. J Biomed Biotechnol. 2011;2011:195483. https://doi.org/10.1155/2011/195483.
Funding
Funding support is provided by the project no. LQ1605 from the National Program of Sustainability II (MEYS CR) and by project FNUSA-ICRC no. CZ.1.05/1.1.00/02.0123 (OP VaVpI). CVD was supported by an NIH T32 training grant (HL007111), European Union Agreement—St. Anne’s University Hospital Brno.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Mayo Clinic owns intellectual property related to the investigated technology reported in the manuscript. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and is being conducted in compliance with Mayo Clinic Conflict of Interest policies.
Ethical approval
The study was approved by the Mayo Clinic Institutional Animal Care and Use Committee (IACUC) and the Ethics Committee of the University of Veterinary and Pharmaceutical Sciences, Brno, Czech Republic.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Killu, A.M., Naksuk, N., Syed, F.F. et al. Feasibility of directional percutaneous epicardial ablation with a partially insulated catheter. J Interv Card Electrophysiol 53, 105–113 (2018). https://doi.org/10.1007/s10840-018-0404-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-018-0404-5