Abstract
Feinberg and Mallatt, in their presentation of neurobiological naturalism, have suggested that visual consciousness was acquired by early vertebrates and inherited by a wide range of descendants, and that its neural basis has shifted to nonhomologous nervous structures during evolution. However, their evolutionary scenario of visual consciousness relies on the assumption that visual consciousness is closely linked with survival, which is not commonly accepted in current consciousness research. We suggest an alternative idea that visual consciousness is linked to a specific class of agency, consequently justifying their phylogenetic claim. We also examine the implication of their phylogenetic claim: visual consciousness is homologous across vertebrates, but its neural basis is not. This apparent incongruence illustrates a general phenomenon of homology, and that the resulting hierarchical view of visual consciousness and its neural basis can be straightforwardly accommodated by neurobiological naturalism. Throughout these discussions, we aim to address the potential theoretical issues in neurobiological naturalism and refine the picture illustrated by Feinberg and Mallatt regarding phylogenetic distribution and trajectories of visual consciousness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
On what type of neural bases is visual consciousness based? Empirical research focusing on humans and monkeys has suggested that the visual neural pathway passing through the thalamus and reaching the primary visual area (V1) of the cerebral cortex is necessary for mammalian visual consciousness, and that other neural pathways, such as those via the superior colliculus, are responsible for unconscious visual processes. In birds, however, a major portion of visual processing is sustained by the neural pathways through the optic tectum, which is homologous to the superior colliculus in mammals. These pathways send signals to the pallium, which is homologous to the mammalian cerebral cortex but has an entirely different neural architecture because it lacks laminar organization. Such visual processing in the optic tectum is also dominant in reptiles, suggesting that the dominance of the optic tectum, not the cortex, is the ancestral condition. Because nonmammalian visual processing is largely based on neural pathways implicated in mammalian unconscious vision, we face the following question: is the subjective aspect of such nonmammalian vision utterly dark like the unconscious vision, such as blindsight?
Feinberg and Mallatt (2016; 2018), who introduced neurobiological naturalism, resist such a corticocentric idea and instead propose that vertebrate sensory consciousness, including vision, has an evolutionary origin in ancestral fish resembling modern lampreys. Sensory consciousness is mainly supported by brain regions distinct from the cerebral cortex in such organisms, and its neural basis has shifted with the evolutionary expansion of the nervous system. In particular, although visual consciousness was originally sustained by the optic tectum in basal vertebrates (fish, amphibians, and reptiles), its “end-site” (Feinberg and Mallatt 2016, 123) has been shifted from the tectum to the pallium (cortex) in birds and mammals. Therefore, they propose that visual consciousness in vertebrates shares an evolutionary origin, but that its neural basis has been phylogenetically diversified.
In proposing their hypothesis, Feinberg and Mallatt have listed the general characteristics of the neural basis of sensory consciousness, which they call “special neurobiological features,” such as multiple neural hierarchies that integrate sensory information and topographically organized neural representations. They use these features as criteria for estimating which organisms are endowed with sensory consciousness. For example, instead of improving the pallium, basal vertebrates have sophisticated brain structures such as the optic tectum, which are responsible for the aforementioned features. Feinberg and Mallatt have surveyed and organized vast empirical literature concerning these features, concluding that not only mammals but also all vertebrates have visual consciousness. This is a novel approach to the following question regarding the distribution of sensory consciousness (Allen and Bekoff 2007; Velmans 2007): how widely distributed is consciousness in various organisms on this planet? Simultaneously, based on their answer to this question, they attempted to identify phylogenetic trajectories of sensory consciousness by proposing the hypothesis that vertebrate sensory consciousness shares a single evolutionary origin but has shifted its neural basis through evolutionary history.
However, their argument seems to be easily disproved by empirical findings concerning unconscious vision. The neural pathway underlying the unconscious visual process, such as blindsight in humans and monkeys, involves the superior colliculus and bypasses cortical area V1. As Feinberg and Mallatt have admitted, this pathway indeed satisfies all the special neurobiological features, such as integrated sensory information in topographical sensory maps. Hence, it can be argued that some cortical contribution is essential for visual consciousness and, therefore, that noncortical visual processing in nonmammalian species remains unconscious as blindsight in humans and monkeys.
In this article, we first discuss this issue. Then, we examine the implications of the evolutionary scenario of visual consciousness depicted by Feinberg and Mallatt. They propose, as mentioned, that the neural basis of visual consciousness has shifted during evolution from optic tectum-centered in basal vertebrates to pallium-centered in birds and mammals. This implies that the underlying neural pathways for visual consciousness are not homologous among these animals, and that visual consciousness itself is homologous. We point out that this apparent incongruence illustrates a general biological phenomenon of homology and argue that this coheres with a central tenet of neurobiological naturalism that consciousness is a higher-level property of the brain. Throughout these discussions, we address the potential theoretical issues in neurobiological naturalism and refine the picture illustrated by Feinberg and Mallatt regarding phylogenetic distribution and trajectories of visual consciousness.
Although we focus on visual consciousness, our discussion might be generalized to other sensory modalities; similar issues concerning pain consciousness in fish have been vigorously debated. Some authors have argued that fish experience pain (Braithwaite 2010; Brown 2015; Sneddon et al. 2014; Sneddon 2011). Others have countered that fish lack a neocortex and, therefore, do not experience pain (Key 2015, 2016; Rose et al. 2014; Rose 2007). Perhaps our present discussion can be naturally extended to such topics, but we limit our discussion to vision because we are able to use much richer empirical details provided by extant studies on visual consciousness.
2 Visual Consciousness in Neurobiological Naturalism
The central visual pathway in humans and monkeys runs through the lateral geniculate nucleus (LGN) in the thalamus to the V1 in the cortex. Although damage to V1 results in a loss of visual consciousness, humans and monkeys with such damage can still discriminate visual stimuli beyond chance (Cowey and Stoerig 1995; Weiskrantz et al. 1974). This phenomenon, called blindsight, is explained by the fact that intact peripheral pathways, bypassing V1, instead go through the superior colliculus to the higher visual areas. It seems that V1 is necessary for visual consciousness, but that the peripheral pathways through the superior colliculus are not. In contrast, birds do not have a neocortex; some portions of their visual neural pathways project to the pallium, which is the region homologous to the mammalian cortex but lacks a layered structure. These pathways consist of several routes, one of which is the lemnothalamic (thalamofugal) pathway, which projects through the principal optic nucleus of the thalamus to a pallial area called the Wulst; another is the collothalamic (tectofugal) pathway, which projects through the optic tectum to the entopallium. These two pathways anatomically correspond to those through the LGN and superior colliculus in mammals, respectively. Interestingly, the collothalamic pathway is the “primary route of visual information” (Wylie et al. 2009, 329) in birds. Damage to this pathway leads to a significant deficit in the ability to discriminate basic visual features such as color and pattern, whereas “lesions in the avian lemnothalamic pathway do not appear to interfere very much with visual ability” (Shimizu and Watanabe 2012, 478; see also Hodos et al. 1984; Nguyen et al. 2004; Parker and Delius 1980; Wakita et al. 1992). These avian neural functions contrast vividly with those of humans and monkeys (for further comparative anatomy, see Butler and Cotterill 2006; Cook et al. 2015; Knudsen 2020; Shimizu et al. 2010; Shimizu and Bowers 1999).
Importantly, mammals, not birds, are “exceptional” in terms of the evolutionary history of vision. The visual pathway relying on the optic tectum is dominant in basal vertebrates such as reptiles. The midbrain comprising the optic tectum is responsible for swift visuomotor control, which has been sophisticated by the relevant pathway extended into the pallium in birds. In contrast, ancestral mammals are nocturnal and rely heavily on olfaction, thus degrading their visual function. When mammals became diurnal and refined their visual system again after the extinction of predatory dinosaurs (Maor et al. 2017), they developed the lemnothalamic pathway, which extends into the visual cortex, such as V1, in humans and monkeys (Gerkema et al. 2013; Heesy and Hall 2010; Knudsen 2020).
As birds appear to have conscious experiences, as many researchers now accept (e.g., Beshkar 2008; Butler et al. 2005; Butler and Cotterill 2006; Griffin and Speck 2004), their visual perceptions should be consciously experienced. If this is the case, then avian visual consciousness should be supported by the collothalamic pathway because of its dominance in the visual behavior of birds, which is responsible for unconscious vision in humans and monkeys. Based on the collothalamic and lemnothalamic pathways in birds and mammals, respectively, it might be thought that visual consciousness was acquired independently. However, this possibility is denied by Feinberg and Mallatt (2016), who argue that visual consciousness has been inherited from ancestral fish, whose neural architecture seems to be well-conserved in modern lampreys, and extended to all vertebrates. In arguing this, they propose that the neural basis of visual consciousness has not only been extended to nonhomologous neural sites between different lineages but has also shifted: “the mammalian superior colliculus is a secondarily simplified [optic] tectum that has lost all or almost all of its original conscious role” (2016, 268, italics added). In other words, neural structures that were once involved in visual consciousness have lost their role over the course of evolutionary history and have been replaced by other neural structures. The rationale they adopt for these claims is that neurobiological features such as topographic sensory maps and multiple neuronal hierarchies are crucial for sensory consciousness. They used these features as neural criteria for sensory consciousness, concluding that the neural pathways relying on the optic tectum support visual consciousness in basal vertebrates.
As they admit, however, the sensory processing that underpins unconscious vision, such as collothalamically sustained blindsight in humans and monkeys, possesses these neurobiological features. “Human sensory hierarchies, which meet our criteria for consciousness […] can perceive stimuli without conscious knowledge, largely subliminally, when the very highest levels of the cerebral cortex are not engaged” (Feinberg and Mallatt 2016, 209). To deal with this problem, they emphasize that visual processing in nonmammalian vertebrates enables their survival, and that humans and monkeys with blindsight are far less likely to survive in natural environments than those with fully functioning visual systems:
Fish process stimuli to a high level that can make fine-grained sensory distinctions necessary for survival. Any human who was dependent on the nonconscious, low-level sensory discriminations that are demonstrated in the laboratory would be almost “senseless” and could not survive if left to his or her own devices. (Feinberg and Mallatt 2016, 210)
Here Feinberg and Mallatt try to justify their argument by assuming a behavioral function associated with visual consciousness that is missing in the mammalian collothalamic vision. However, their idea linking survival and visual consciousness is not widely accepted and seems to contradict the empirical findings derived from neuropathological studies. It has been reported that a patient with a deficit in the ventral visual pathway cannot see shapes or identify visual objects but can walk while avoiding obstacles (Mestre et al. 1992; Milner and Goodale 2008). Furthermore, a patient with hemineglect in her left visual field failed to discriminate two pictures of a house, where one of them exhibited a fire on the left side of the house; however, when asked which house she would prefer to live in, she reliably chose the picture without fire (Marshall and Halligan 1988). These findings suggest that unconscious vision is sensitive to visual information related to survival; therefore, they seem to falsify Feinberg and Mallatt’s idea concerning behavioral functions of visual consciousness. Although this falsification has not been fully established, it is fair to say that the alleged link between visual consciousness and survivability does not appear convincing.
Furthermore, corticocentrists, who insist that visual consciousness requires the visual cortex, would emphasize that the crucial criterion is not survivability, but rather reportability. It has sometimes been explicitly argued that reportability is the “gold standard” of conscious perception (Marcel 1988; Naccache 2006; Brown et al. 2019). In line with this, it could be argued that the neural basis of visual consciousness should be specified within organisms with reportability, such as humans and monkeys, and then extrapolated to other species. Because cortical areas including V1 are implicated in the visual consciousness of humans and monkeys, we should conclude that visual consciousness is limited to organisms with a well-developed cortex, that is, mammals, or so the corticocentrists would suggest.Footnote 1
Nevertheless, we do not consider these objections to be disproving of the phylogenetic distribution and trajectories of visual consciousness portrayed by Feinberg and Mallatt. We suggest an alternative to survivability and introduce it to remedy the inference of visual consciousness in nonmammalian vertebrates, consequently justifying the estimated evolutionary scenario of visual consciousness and the neural basis. To this end, we begin by taking a closer look at the nature of reportability.
3 Agency and Visual Consciousness: An Alternative Link
Corticocentrists could argue that reportability is the gold standard of consciousness, as mentioned, but what exactly is the behavioral function that underpins reportability? Supposedly, it is the ability to perform communicative speech, which is manifested in interactions between experimenters and experimental subjects (Dennett 1991). Another view indicates that the human act of reporting involves self-monitoring and finds a constitutive link between consciousness and higher-order thought (Lau and Rosenthal 2011; Rosenthal 2002). However, what is customarily called reportability in consciousness research does not actually require such abilities. For example, monkeys lack the capacity for verbal communication and higher-order thought, but they can still “report” visual stimuli by, for example, appropriately pressing buttons. As Baars and Edelman (2012) point out:
Accurate, voluntary report is still our most useful behavioral index, though new brain measures are emerging. Non-human animals can be trained to “report” conscious stimuli using a method called ‘matching to sample.’ That is, an animal can be trained to point to a Munsen color chip that matches the color of a sample stimulus. (p. 292)
Therefore, generally speaking, the reportability adopted in consciousness research consists of the ability to act in appropriate accordance with sensory stimuli.
Although we do not deny that verbal communication and self-awareness can be important behavioral markers in the study of consciousness, especially in humans, we should not overlook the fact that those abilities are premised on their dependence on agency. On the one hand, verbal communication depends on the background intention to convey relevant information. As Dennett (1991) observes, “[w]hich speech act a particular button-pushing can be taken to execute depends on the intentional interpretation of the interactions between subject and experimenter that were involved in preparing the subject for the experiment” (p. 77). On the other hand, sensory self-awareness requires effortful attention to sensory features and recognizing them as features of experience rather than as features of external stimuli. In Ryle’s (1949) words, “introspection is an attentive operation” (p. 164) (see also Jack and Shallice 2001; Nelkin 1995; Rosenthal 1986; Schmitz and Johnson 2007). These facts suggest that reportability always involves agency, even in humans.
In contrast to these ideas, it is sometimes argued that agency does not distinguish conscious from unconscious perception (e.g., Rosenthal 2008; Brown et al. 2019). These discussions commonly refer to blindsight, which involves patients without visual awareness successfully responding with their behavior to a variety of visual information, suggesting that unconscious perception is sufficient for visual agency. These findings, according to the opponents, demonstrate the dissociation of agency and conscious vision.
However, this objection overlooks the difference between agency and mere bodily movement or its available control. To clearly distinguish agency involving consciousness from that which operates without it, here we focus on a specific class of agency that involves sensitivity to the organism’s reasons and purposes. This class of agency is absent in the visual behavior of the type manifested in blindsight. As has often been pointed out, the visual behavior of blindsight has to be prompted by the experimenter, and the relevant unconscious visual information is manifested only in forced judgment tasks; therefore, it lacks sensitivity to the patient’s own reasons. For example, patients may be thirsty but will not reach for a glass of water in their blinded visual field (Marcel 1986; similarly, Flanagan 1992; Van Gulick 1989; Young 2006). Furthermore, deficit studies have demonstrated the association between consciousness and agency. Milner and Goodale’s patient called “DF,” who sustained damage to the ventral visual stream, could manually grasp objects with shapes that she never visually recognized with the intact dorsal visual stream. However, her grasping loses meaningfulness:
When she [DF] reaches out to pick up such objects (ones she cannot identify by sight), her grasp is perfectly matched to the object’s size, shape, and orientation, but shows no indication that she understands its function. Thus, she grasps the screwdriver by its shaft rather than its handle––and only then rotates it in her hand so that she can hold it properly. (Goodale and Milner 2005, 107)
With such observations, Goodale and Milner concluded: “The ventral stream makes contributions to action in several ways. For example, it is the ventral stream that identifies the goals for action, which enables the brain to select the class of action to perform” (p. 107). Therefore, visual consciousness seems to function as a background for such agency, and the relevant link has been termed by Clark (2001) as “experience-based selection” (see also Bayne 2013; Clark 2007; 2009; Dretske 2006).
Although the nature of agency has sometimes been overintellectualized by philosophers as requiring conceptual capacities or reflective awareness and has been considered absent in animals (e.g., Hacker 2007; McDowell 1994), we do not focus on such a demanding class of agency. It is fair to say that actions in general are initiated based on their reasons or purposes, as mentioned, and this class of agency is clearly manifested by more “primitive” organisms than Homo sapiens living with much less conceptual capacity.Footnote 2 Although it is certainly a significant philosophical challenge to accurately define the nature of agency, we only have to specify the minimal conception of agency that is linked with visual consciousness, such as being sensitive to internal goals beyond external stimuli. That is, an organism’s behavior manifests agency when it does not respond to stimuli in a fixed manner and instead behaves in a way that is linked to a variable purpose.Footnote 3
The link between visual consciousness and minimal agency enables us to justify the relevant phylogenetic distribution of visual consciousness. The collothalamic pathway, which is responsible for unconscious vision in humans, is well-developed and “involved in visual information processing, beyond mechanical visual reflexes” in birds (Shimizu and Watanabe 2012, 475). Additionally, birds use the latter portion of this pathway (i.e., after passing through the optic tectum) so that it “analyzes output of the tectum more slowly and carefully than the tectum [itself] and supports sustained and deliberate behaviors” (p. 480). We consider this the case for the aforementioned class of agency, which, in conjunction with its link to visual consciousness, justifies attributing visual consciousness to the collothalamic pathway in birds. The same reasoning applies to other vertebrate species. According to Feinberg and Mallatt, “[e]ven lampreys have complex behavioral adaptations, dependent on vision and other senses, that far exceed what could be produced by human paradigms of unconscious perception” (Feinberg and Mallatt 2013, 18). This statement can be easily interpreted as attributing visual consciousness to fish based on their agency.Footnote 4
Our focus on agency also explains the intuition behind Feinberg and Mallatt’s appeal to survivability. Feinberg and Mallatt assume that visual consciousness should have an adaptive value and, thus, functions in a way that fulfills biological purposes such as survival. (Remember their statement that “[a]ny human who was dependent on the nonconscious, low-level sensory discriminations that are demonstrated in the laboratory […] could not survive if left to his or her own devices” [Feinberg and Mallatt 2016, 210].) This assumption is partially true because vertebrate organisms would exercise agency to fulfill biological purposes such as getting foods, avoiding danger, finding partners, and others, and––as we have claimed––visual consciousness would function as a background to this class of agency. Therefore, to this extent, visual consciousness appears to contribute to survivability. However, such a contribution to survivability has a clear limit. This is because, especially in highly intelligent organisms such as humans, the purposes of action can deviate from biological ones. An oft-cited example is sexual intercourse with contraception, which is “an act which serves the vehicle’s goal of pleasure without serving the gene’s goal of reproduction” (Stanovich and West 2000, 712; see also Boudry et al. 2015; Stanovich 2005). There are many examples of civilized human behavior that fulfill a wide variety of purposes and reasons that are biologically insignificant. Therefore, information and knowledge obtained through conscious visual perception are not necessarily used for survival or reproduction, but rather for the organism’s own purposes, whatever they may be. In this manner, although visual consciousness plays the role of the background of agency, the relevant purposes are not necessarily biological. We suspect that this also explains why most consciousness studies, now heavily dependent on human subjects, do not use survivability as any working criterion for consciousness.
In summary, the current assumption involved in consciousness research is that reportability acts as the gold standard to attribute consciousness, but it does not exclude the link between agency and visual consciousness. This link is also supported by empirical findings concerning visual consciousness in humans and monkeys. Furthermore, this link works as an alternative to Feinberg and Mallatt’s emphasis on survivability and justifies their hypothesis about the phylogenetic distribution of visual consciousness.
4 Homologous Visual Consciousness Underpinned by Nonhomologous Neural Basis
As noted, according to Feinberg and Mallatt, visual consciousness is a biological trait inherited from ancestral fish and is thus homologous throughout extant vertebrates. In contrast, the underlying neural pathways of mammalian and avian visual consciousness are nonhomologous (mainly lemnothalamic and collothalamic pathways, respectively). Therefore, their idea seems to involve a claim of multiple realizations because the neural basis of visual consciousness has shifted to different (i.e., nonhomologous) neural sites across the relevant species.Footnote 5 However, multiple realizations in biology typically involve convergent evolution, such that resembling characteristics are acquired by independent evolutionary processes. If, as Feinberg and Mallatt suggested, the visual consciousness of vertebrates was acquired in their common ancestor and then inherited during evolution, vertebrate visual consciousness is not a product of convergent evolution. Therefore, their view seems to imply a tricky proposition: visual consciousness is a homologous trait across vertebrate species, but not if viewed with its neural underpinnings.Footnote 6
It could be argued that such a disparity is apparent and can be explained away because, generally speaking, homology can only be described as homologous as something. For instance, the wings of bats and those of birds are not homologous as wings (i.e., as a membranous structure for flight); the wings of bats were acquired after the mammals diverged from the lineage leading to birds. However, these wings are homologous as forelimbs; mammals and birds share a common ancestor in the ancestral tetrapod vertebrates and maintain the tetrapod body plan. Therefore, how a trait is specified can affect whether it is homologous or not, which is generally recognized.Footnote 7 Visual consciousness in mammals and birds might be addressed in a similar manner. Indeed, Feinberg and Mallatt seem to suggest such an explanation: visual consciousness has become involved in the cerebral cortex and the pallium, respectively, in mammals and birds, for sophisticated functions such as visual memory, learning, and attention. Such higher-level functioning visual consciousness is speculated to be an adaptation to similar ecological challenges and, thus, “a case of convergent evolution” (2016, 125, emphasis in original). Therefore, one might think that visual consciousness is nonhomologous between mammals and birds.
This appears plausible but, in fact, it is not. The issue here is with the phenomenal consciousness––what it is like to see something––as Feinberg and Mallatt, who distinguished the phenomenal sense of visual consciousness from the cognitively refined type of visual consciousness, explicitly acknowledge. As they have argued, the phenomenal sense of vertebrate visual consciousness has its origin in early vertebrate animals, but it has shifted to differently developed neural pathways across species through phylogenetic branches. Therefore, homologous consciousness with a nonhomologous neural basis cannot be explained away.Footnote 8 Instead, we believe that it is possible to accommodate this dissociation in homology as a real phenomenon. The key is the hierarchical-relative character of homology.Footnote 9
The customary classes of phenotypic features to which the concept of homology applies are morphological and anatomical. For example, tetrapod fingers are homologous among species in their clade, but the mechanism of their development is not necessarily conserved. In the majority of tetrapod vertebrates, including mammals and birds, fingers are formed by programmed cell death (apoptosis) in the tissue between the finger primordia; however, in urodele amphibians, they are formed as each finger primordium grows outward (Hall 2003). Another well-known example is the development of the vulva in nematodes. In Caenorhabditis elegans, the vulva is achieved through specific fusion of the 12 precursor cells with subcutaneous tissue; however, in Pristionchus pacificus, cell fusion is replaced by apoptosis (Félix 2005; Sommer 2008). These phenomena indicate that the developmental mechanisms of homologous morphological features are not conserved during their evolution. This type of evolutionary change in developmental mechanisms is known as “developmental system drift” (Haag and True 2018; Müller 2003; True and Haag 2001).
This diversification of developmental systems can be accompanied by conservation and variation at the molecular level. In the case of the aforementioned nematode vulva, a homologous gene, lin-39, is involved in both nematode species, and its mutation results in the failure of vulva formation through unsuccessful cell fusion in C. elegans and unsuccessful apoptosis in P. pacificus. However, in contrast to this molecular commonality, Wnt signaling is required only in C. elegans; it suppresses the developmental process in P. pacificus (Félix 2005). Based on these observations, Sommer (2008) said, “[t]he vulva is a homologous organ at the morphological level, it is generated by homologous precursor cells, but requires, in part, different cell–cell interactions, non-homologous regulatory networks and molecular modules” (p. 657).
Such molecular conservation and variation have been observed in many other developmental processes as well. For example, fruit flies (Drosophila) and houseflies (Musca) share the architecture of sex determination mechanisms, which comprise the “key genes” sensitive to a primary genetic signal and its downstream cascades involving the “double-switch genes.” However, the only homologous gene functioning therein is dsx, which plays the role of the double-switch genes, and the rest are carried by nonhomologous genes (Schütt and Nöthiger 2000). Such molecular variability is also observed in cell division, which is the most basic process of development and “clearly homologous between all species” (Striedter 2019, 64). The molecular signaling pathway controlling the G1–S phase of cell division consists of multiple molecular feedback interactions whose network topology is widely shared from yeasts to mammals. Nevertheless, the majority of relevant genes and proteins have lost sequence similarity between species and, therefore, are not considered homologous (Cross et al. 2011). Such a phenomenon is called “deep non-homology” by Striedter (2019), who states:
[…] [T]raits can maintain their phylogenetic identity as lower-level elements drop out or new ones are added into their causal nexus […]. The key idea is that several different constellations of lower-level mechanisms are capable of giving rise to the higher-level trait, and evolution can move smoothly between them. (p. 66, italics added)
Ereshefsky (2009) expresses the same point with the notion of hierarchical disconnect: “[n]onhomologous factors at one level can cause homologous traits at a higher hierarchical level” (p. 226) (see also Ereshefsky 2012). Similarly, according to Müller (2003), “homology denotes constancy of constructional organization despite changes in underlying generative mechanisms” (p. 64). According to Sommer (2008), “there is a potential uncoupling of homology at different hierarchical levels of biological organization” (p. 653).
To fully unpack the implication of this aspect of homology, we emphasize that the attribution of homology applies to not only morphological and anatomical features but also behavioral and psychological traits, and this has been recognized during recent research of homology (Ereshefsky 2007; Hall 2013; Powell and Shea 2014; Rendall and Di Fiore 2007; Suzuki and Tanaka 2017).
Behavioral homology has been discussed by ethologists (see Lorenz 1958; 1974 as pioneering works) and is now subject to quantitative analysis. For example, ancestral states of behavior have been estimated using the maximum parsimony method, which is the same method used for investigating morphological traits (e.g., Lin et al. 2004; Price et al. 2004). One interesting case study is the flexion movement of the nudibranch molluscs, which swim using left–right flexion and dorsal–ventral flexion. Among them, a clade including Melibe and Dendronotus exhibits left–right body flexion, suggesting that this behavior is homologous in this clade (Newcomb et al. 2012; Sakurai and Katz 2017). However, it has also been found that this homologous behavior is realized by quite different and evolutionarily changed neural connections (Sakurai et al. 2011; Sakurai and Katz 2017), and this seems to be a case of the hierarchical disconnect.
The homology of a psychological trait such as vision has also been discussed (e.g., Matthen 2007; 2015). For example, although a wide variety of differences exist in the visual functions of mammals and birds, they are still homologous traits derived from a common reptile ancestor. Birds moved their habitat to the sky, which led to special modifications to their visual functions, such as sun compass navigation. However, ancestral mammals evolved into nocturnal animals; therefore, many of their descendants possessed poor color vision. Nevertheless, as primates adapted to arboreal life, they secondarily evolved color vision that allowed them better ability to distinguish colorful fruits from the green background. Despite these large differences, there is a sense that vision is homologous between birds and mammals because it is inherited from the common ancestor.
The homology of vision can be correctly evaluated only relative to its specific aspects, as we have noted, especially given its functional divergence. To illustrate this, we consider the well-known case of color vision in more detail (reviewed in Bowmaker 2008; Hunt et al. 2009; Thoreson and Dacey 2019, etc.). Tetrachromatic vision in most vertebrates is sustained by four types of visual pigments: red opsin, green opsin, blue opsin, and ultraviolet opsin. However, nocturnal mammals have shifted from tetrachromatic vision to dichromatic vision due to the loss of green and blue opsins. Old world monkeys, which are a group of primates, later returned to their diurnal lifestyle and “reinvented” another green opsin through gene duplication and differentiation of the red opsin. Therefore, they evolved trichromatic vision. According to these well-known findings, the trichromatic color vision of birds and that of primates do not have a common origin. They inherited color vision from the common ancestor; however, more finely, in terms of trichromatic color vision, they did not inherit it from the common ancestor. This is parallel to the situation where the wings of a bird and those of a bat have been inherited from a common ancestor as forelimbs, but are not homologous as wings. Similarly, homology of vision can be evaluated relative to its specific functional aspects.
We argue that the phenomenal aspect of vision can also be the basis for considering its homology. Although there are numerous differences in optic information delivered by vision in birds and primates, as described, the information reflected in visual consciousness shares a common aspect: what it is like to see. Birds and primates have inherited this aspect of vision from a common ancestor. According to Feinberg and Mallatt’s phylogenetic hypothesis, visual consciousness was acquired by ancestral vertebrates; therefore, their visual consciousness is homologous. The optical information reflected in the visual consciousness would have changed with the evolutionary transformation of the visual organs and the nervous systems related to them. Nevertheless, visual consciousness, in the sense of enjoying the relevant information from a first-person visual perspective, has been inherited from the common ancestor by birds and primates.
If visual consciousness is a homologous psychological trait between birds and primates, then there is no contradiction when saying that its neural basis is nonhomologous between them. This can be understood as a case of hierarchical disconnect; the homology of visual consciousness is specified at the “higher” psychological level, whereas the homology of its neural basis of visual consciousness is specified at the “lower” morphological–anatomical level. Therefore, patterns such as the evolutionary shift of the neural basis from the optic tectum to the visual cortex and the reversal of the neural pathways responsible for visual consciousness between birds and primates are accommodated as instantiating the general phenomena in the biological order.
It might be wondered whether consciousness can be regarded as a homologous trait. However, our focus on consciousness appears dubious only when consciousness is assumed to be something beyond biological. As long as visual consciousness has some biological nature, as presumed by neurobiological naturalism, there is no obstacle in considering it a homologous trait. Furthermore, neurobiological naturalism provides a natural background for the hierarchical conception of visual consciousness and its neural basis. In the underlying ontology of neurobiological naturalism, consciousness is one of the“ordinary higher-level biological properties” (Searle 1992, 28) and an “evolved phenotypical trait of certain types of organisms with highly developed nervous system” (p. 90), in an analogous manner, with water being a higher-level property of aggregates of H2O molecules. This means that consciousness is at a level higher than, and therefore different from, the level of its neural basis. (Compare this with the case of identity, for which there is no difference in levels, such as Phosphorus and Hesperus.) Therefore, the hierarchical disconnect between visual consciousness and its neural basis, which is found when evaluating their homology from the evolutionary perspective, has a natural home in neurobiological naturalism.
5 Concluding Remarks
We have argued that visual consciousness is associated with a particular class of agency. This association is not only consistent with the practice of consciousness studies using reportability but also supported by empirical findings about visual consciousness in humans and monkeys. This association counters the corticocentrist view and justifies Feinberg and Mallatt’s view of the distribution and trajectories of visual consciousness and its neural basis. We have also examined the implications of their view in terms of homology, suggesting that visual consciousness and its neural basis are at different levels of homology, thus making a case for hierarchical disconnect. This picture can be straightforwardly incorporated into neurobiological naturalism, where visual consciousness is a higher-level feature of the brain, bringing us a novel perspective on the inheritance and diversification of visual consciousness and its neural basis in vertebrates.
In contrast with existing evolutionary approaches to consciousness that have often been pursued along with the question of whether consciousness is an evolutionary adaptation (e.g., James 1890; more recently, for example Polger 2017; Robinson et al. 2015; Robinson 2007), we have limited ourselves to more phenomenological descriptions by focusing on the superficial dynamics of the target phenomenon (Suzuki and Tanaka 2017), which is, in our case, the evolutionary trajectories of visual consciousness and its neural basis. Our work may be a case of “lineage explanation” (Calcott 2009), which has rarely been addressed during evolutionary inquiries of consciousness. Such an explanation is distinct from selective explanations focusing on any adaptive character of consciousness because the evolutionary trajectories of consciousness and its neural basis can be considered independently of causal factors behind them. However, given the link between agency and consciousness, it can be inferred that consciousness brings some adaptive advantage for most situations except for cases where, as we have explained, the organisms’ purposes are dissociated from biological fitness; it might be further argued that there was selection for consciousness in its origin. Arguing so requires us to consider various counter-possibilities and bind together findings from neuroscience, behavioral studies, evolutionary biology, and consciousness studies, which is challenging and interesting.
Moreover, we have left Feinberg and Mallatt’s metaphysical claims regarding the hard problem of consciousness unexamined (Chalmers 1996). While rejecting the anti-physicalist view of consciousness, they argue that consciousness cannot be fully reduced to the structure and function of the nervous system. Their version of nonreductive physicalism, however, probably inherits many problematic features of its philosophical root, Searle’s biological naturalism (Collins 1997; Corcoran 2001; Kim 1995). Nevertheless, Feinberg and Mallatt’s view of the evolution of visual consciousness can be considered in isolation from such issues. In this paper, drawing on their work, we have attempted to make sense of the phylogenetic distribution and trajectories of visual consciousness in a more coherent manner, illustrating its implications for the positions of visual consciousness and its neural basis within the natural order.
Change history
10 November 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10838-022-09627-0
Notes
For further arguments in favor of agency without conceptual abilities, see Hurley (2003), Glock (2009), and others. Some scholars with naturalistic tendency have proposed the notion of agency that applies to an overly broad range of biological behaviors, including bacterial chemotaxis (e.g., Barandiaran et al. 2009; Barham 2012; Fulda 2017). However, such considerations have emphasized the embodied character of biological behaviors such as sensorimotor coordination and close coupling with the environment, which is distinct from the notion of agency we are engaged in here. Such an embodied class of agency is rather typical of visual behaviors based on human dorsal visual pathways, which are known to be dissociable from visual consciousness (Clark 2007; 2009; Foley et al. 2015; Kozuch 2015; see also Milner 2012; Milner and Goodale 2008).
An important concern is that our notion of agency is too permissive. For example, slime mold is known to show a variety of apparently intelligent behavior involving problem solving and learning, and may be thought of as manifesting agency (we owe this case to an anonymous referee); it may be, therefore, argued that our focus on agency could admit of consciousness in slime mold. However, first, it is doubtful that slime mold has anything worth to call an “internal” goal which typically operates within a cognitively organized biosystem. Second, the supposed link between agency and consciousness is premised on the thesis that consciousness is representational; that is, neural representations implemented in the brain are conscious when they are involved in organisms’ exercising agency. This representational character is stated as “isomorphically (topographically) mapped neural representations” by Feinberg and Mallatt (2016, 129) and listed in the neurobiological features crucial for consciousness. This character is absent in slime mold’s intelligent behavior, which has been indeed discussed as a case for non-representational cognition (e.g., Walmsley 2020). We, therefore, think that alleged cases of nonconscious agency fail to satisfy the representational basis of consciousness or are actually engaged with inappropriate classes of agency (see also footnote 2).
Such descriptions of agency can be found in many discussions in favor of the existence of sensory consciousness, such as pain, in a wide range of animal species, that emphasize flexible, complex, and adaptive behaviors (e.g., Braithwaite 2010; Brown 2016; Godfrey-Smith 2016a; Mikhalevich and Powell 2020; Powell 2020; Sneddon et al. 2014; Sneddon 2003; 2011).
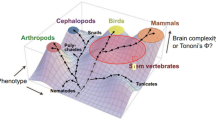
We have focused on the visual consciousness of vertebrates in this article; it is homologous across vertebrate species. Interestingly, it has been argued that invertebrates, such as some cephalopods and arthropods, have visual consciousness, as some researchers, including Feinberg and Mallatt, argue (cf. Ginsburg and Jablonka 2019; Godfrey-Smith 2016b; Tye 2017; Powell 2020). If this is the case, visual consciousness will be a product of convergent evolution; that is, visual consciousness has evolved independently multiple times (at least three times) in different lineages. Given this assumption, our present discussion implies further “distance” between vertebrate and invertebrate visual consciousnesses, because they are non-homologous both in the phenomenal sense and in its neural basis. For existing discussions on the (non-)homology of consciousness between vertebrates and invertebrates, see Mikhalevich and Powell (2020 and Powell (2020).
One may think that the present case is not convergence but parallelism, where a feature is present in closely related organisms but not present continuously in all members of the lineage or in the most recent common ancestor of the two organisms/groups being compared (Hall 2012). Furthermore, it might be argued that parallelism implies homology in virtue of developmental bases (Ramsey and Peterson 2012), in which case one could suggest that both visual consciousness and its neural basis are homologous between birds and mammals, attempting to explain away the discrepancy in homology. However, first, it is controversial that parallelism implies homology (Currie 2014; McConwell and Currie 2017); second, in any case, the higher-level functioning visual consciousness is based on different neural mechanisms which do not share the developmental bases between birds and mammals.
It should be noted that the homology concept itself has been a matter of intense debate in both biology and philosophy (briefly reviewed in Suzuki and Tanaka 2017). Whereas homologous characters were regarded to form a type (the typological concept of homology), in neo-Darwinian evolutionary theory where typological notions are largely criticized, historical continuity has been required for homology (the historical concept of homology). However, the advance of evolutionary developmental biology (EvoDevo) led many new concepts of homology, which resist the historical concept and focus much more on the developmental mechanisms (e.g., Abouheif 1997; Müller 2003; 2010; Roth 1984; 1991; Shubin et al. 1997; 2009; Wagner 1989; 2014) and has sometimes led to suggestions that homology is widespread than considered otherwise (e.g. Ramsey and Peterson 2012). We think that our present discussion drawing on the hierarchical-relative character of homology makes sense regardless of which concept of homology should be adopted (see also footnote 8). Nevertheless, given this complexity of the debate, it is interesting to scrutinize whether and how homology concepts could affect the phylogeny of consciousness and its neural bases, which is not further pursed in the present article.
References
Abouheif, E. 1997. Developmental genetics and homology: A hierarchical approach. Tree 12: 405–408.
Allen, C., and M. Bekoff. 2007. Animal consciousness. In The Blackwell companion to consciousness, eds. M. Velmans, and S. Schneider, 58–71. Oxford: Blackwell.
Baars, B. J., and D. B. Edelman. 2012. Consciousness, biology and quantum hypotheses. Physics of Life Reviews 9 (3): 285–294.
Barandiaran, X. E., E. Di Paolo, and M. Rohde. 2009. Defining agency: Individuality, normativity, asymmetry, and spatio-temporality in action. Adaptive Behavior 17 (5): 367–386.
Barham, J. 2012. Normativity, agency, and life. Studies in History and Philosophy of Biological and Biomedical Sciences 43 (1): 92–103.
Bayne, T. 2013. Agency as a marker of consciousness. In Decomposing the will, eds. A. Clark, J. Kiverstein, T. Vierkant, and T. Vierkant, 160–181. Oxford: Oxford University Press.
Beshkar, M. 2008. Animal consciousness. Journal of Consciousness Studies 15 (3): 5–33.
Boudry, M., M. Vlerick, and R. McKay. 2015. Can evolution get us off the hook? Evaluating the ecological defence of human rationality. Consciousness and Cognition 33: 524–535.
Bowmaker, J. K. 2008. Evolution of vertebrate visual pigments. Vision Research 48 (20): 2022–2041.
Braithwaite, V. 2010. Do fish feel pain? Oxford: Oxford University Press.
Brown, C. 2015. Fish intelligence, sentience and ethics. Animal Cognition 18 (1): 1–17.
Brown, C. 2016. Fish pain: An inconvenient truth. Animal Sentience 3: 32.
Brown, R., H. Lau, and J. E. LeDoux. 2019. Understanding the higher-order approach to consciousness. Trends in Cognitive Sciences 23 (9): 754–768.
Butler, A. B., and R. M. Cotterill. 2006. Mammalian and avian neuroanatomy and the question of consciousness in birds. The Biological Bulletin 211 (2): 106–127.
Butler, A. B., P. R. Manger, B. I. Lindahl, and P. Århem. 2005. Evolution of the neural basis of consciousness: A bird–mammal comparison. BioEssays 27 (9): 923–936.
Calcott, B. 2009. Lineage explanations: Explaining how biological mechanisms change. The British Journal for the Philosophy of Science 60 (1): 51–78.
Chalmers, D. J. 1996. The conscious mind: In search of a fundamental theory. Oxford: Oxford University Press.
Clark, A. 2001. Visual experience and motor action: Are the bonds too tight? Philosophical Review 110 (4): 495–519.
Clark, A. 2007. What reaching teaches: Consciousness, control, and the inner zombie. The British Journal for the Philosophy of Science 58 (3): 563–594.
Clark, A. 2009. Perception, action, and experience: Unraveling the golden braid. Neuropsychologia 47 (6): 1460–1468.
Collins, C. 1997. Searle on consciousness and dualism. International Journal of Philosophical Studies 5 (1): 15–33.
Cook, R. G., M. A. J. Qadri, and A. M. Keller. 2015. The analysis of visual cognition in birds: Implications for evolution, mechanism, and representation. Psychology of Learning and Motivation 63: 1–38.
Corcoran, K. 2001. The trouble with Searle’s biological naturalism. Erkenntnis 55 (3): 307–324.
Cowey, A., and P. Stoerig. 1995. Blindsight in monkeys. Nature 373 (6511): 247–249.
Cross, F. R., N. E. Buchler, and J. M. Skotheim. 2011. Evolution of networks and sequences in eukaryotic cell cycle control. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 366 (1584): 3532–3544.
Currie, A. M. 2014. Venomous dinosaurs and rear-fanged snakes: homology and homoplasy characterized. Erkenntnis 79 (3): 701–727.
Dennett, D. 1991. Consciousness explained. Boston: Little, Brown, and Co.
Dretske, F. 2006. Perception without awareness. In Perceptual experience, eds. T. S. E. Gendler, and J. E. Hawthorne, 147–189. Oxford: Oxford University Press.
Edelman, D. B. 2016. Leaving the door open for fish pain: Evolutionary convergence and the utility of “just-so stories”. Animal Sentience, 2016.062.
Ereshefsky, M. 2007. Psychological categories as homologies: Lessons from ethology. Biology and Philosophy 22 (5): 659–674.
Ereshefsky, M. 2009. Homology: Integrating phylogeny and development. Biological Theory 4 (3): 225–229.
Ereshefsky, M. 2012. Homology thinking. Biology and Philosophy 27 (3): 381–400.
Feinberg, T. E., and J. Mallatt. 2013. The evolutionary and genetic origins of consciousness in the Cambrian period over 500 million years ago. Frontiers in Psychology 4: 667.
Feinberg, T. E., and J. M. Mallatt. 2016. The ancient origins of consciousness: How the brain created experience. Cambridge, MA: MIT Press.
Feinberg, T. E., and J. M. Mallatt. 2018. Consciousness demystified. Cambridge, MA: MIT Press.
Félix, M. A. 2005. An inversion in the wiring of an intercellular signal: Evolution of Wnt signaling in the nematode vulva. BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology 27 (8): 765–769.
Flanagan, O. J. 1992. Consciousness reconsidered. Cambridge, MA: MIT Press.
Foley, R. T., R. L. Whitwell, and M. A. Goodale. 2015. The two-visual-systems hypothesis and the perspectival features of visual experience. Consciousness and Cognition 35: 225–233.
Fulda, F. C. 2017. Natural agency: The case of bacterial cognition. Journal of the American Philosophical Association 3 (1): 69–90.
Gerkema, M. P., W. I. Davies, R. G. Foster, M. Menaker, and R. A. Hut. 2013. The nocturnal bottleneck and the evolution of activity patterns in mammals. Proceedings of the Royal Society of London B: Biological Sciences, 280(1765), 20130508.
Ginsburg, S., and E. Jablonka. 2019. The evolution of the sensitive soul: Learning and the origins of consciousness. Cambridge, MA: MIT Press.
Glock, H. J. 2009. Can animals act for reasons? Inquiry : a journal of medical care organization, provision and financing 52 (3): 232–254.
Godfrey-Smith, P. 2016a. Pain in parallel. Animal Sentience, 3 (21).
Godfrey-Smith, P. 2016b. Other minds: The octopus, the sea, and the deep origins of consciousness. New York: Farrar, Straus and Giroux.
Goodale, M., and D. Milner. 2005. Sight unseen: An exploration of conscious and unconscious vision. Oxford: Oxford University Press.
Griffin, D. R., and G. B. Speck. 2004. New evidence of animal consciousness. Animal Cognition 7 (1): 5–18.
Haag, E. S., and J. R. True. 2018. Developmental system drift. In Evolutionary developmental biology: A reference guide, eds. L. Nuño, G. B. de la Rosa, and Müller, 1–12. Cham: Springer.
Hall, B. K. 2003. Descent with modification: The unity underlying homology and homoplasy as seen through an analysis of development and evolution. Biological Reviews of the Cambridge Philosophical Society 78 (3): 409–433.
Hall, B. K. 2012. Parallelism, deep homology, and evo-devo. Evolution & Development 14 (1): 29–33.
Hall, B. K. 2013. Homology, homoplasy, novelty, and behavior. Developmental Psychobiology 55 (1): 4–12.
Heesy, C. P., and M. I. Hall. 2010. The nocturnal bottleneck and the evolution of mammalian vision. Brain, Behavior and Evolution 75 (3): 195–203.
Hodos, W., K. A. Macko, and B. B. Bessette. 1984. Near-field acuity changes after visual system lesions in pigeons. II. Telencephalon. Behavioural Brain Research 13 (1): 15–30.
Hunt, D. M., L. S. Carvalho, J. A. Cowing, and W. L. Davies. 2009. Evolution and spectral tuning of visual pigments in birds and mammals. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 364 (1531): 2941–2955.
Hacker, P. M. S. 2007. Human nature: The categorical framework. Oxford: Basil Blackwell.
Hurley, S. 2003. Animal action in the space of reasons. Mind and Language 18 (3): 231–257.
Jack, A. I., and T. Shallice. 2001. Introspective physicalism as an approach to the science of consciousness. Cognition 79 (1–2): 161–196.
James, W. 1890/1981. The principles of psychology. Cambridge: Harvard University Press.
Key, B. 2015. Fish do not feel pain and its implications for understanding phenomenal consciousness. Biology and Philosophy 30 (2): 149–165.
Key, B. 2016. Why fish do not feel pain. Animal Sentience 3: 1.
Kim, J. 1995. Mental causation in Searle’s “Biological Naturalism”. Philosophy and Phenomenological Research 55 (1): 189–194.
Knudsen, E. I. 2020. Evolution of neural processing for visual perception in vertebrates. Journal of Comparative Neurology 528 (17): 2888–2901.
Kozuch, B. 2015. Dislocation, not dissociation: The neuroanatomical argument against visual experience driving motor action. Mind and Language 30 (5): 572–602.
Lau, H., and D. Rosenthal. 2011. Empirical support for higher-order theories of conscious awareness. Trends in Cognitive Sciences 15 (8): 365–373.
Lin, C. P., B. N. Danforth, and T. K. Wood. 2004. Molecular phylogenetics and evolution of maternal care in Membracine treehoppers. Systematic Biology 53 (3): 400–421.
Lorenz, K. Z. 1958. The evolution of behavior. Scientific American 199 (6): 67–78.
Lorenz, K. Z. 1974. Analogy as a source of knowledge. Science 185 (4147): 229–234.
Manzotti, R. 2016. No evidence that pain is painful neural process. Animal Sentience 3: 11.
Maor, R., T. Dayan, H. Ferguson-Gow, and K. E. Jones. 2017. Temporal niche expansion in mammals from a nocturnal ancestor after dinosaur extinction. Nature Ecology and Evolution 1 (12): 1889–1895.
Marcel, A. 1988. “Phenomenal experience and functionalism”. In Consciousness in modern science, eds. A. Marcel, and E. Bisiach, 121–158. Oxford: Oxford University Press.
Marcel, A. J. 1986. Consciousness and processing: Choosing and testing a null hypothesis. Behavioral and Brain Sciences 9 (1): 40–41.
Marshall, J. C., and P. W. Halligan. 1988. Blindsight and insight in visuo-spatial neglect. Nature 336 (6201): 766–767.
Matthen, M. 2007. Defining vision: What homology thinking contributes. Biology and Philosophy 22 (5): 675–689.
Matthen, M. 2015. The individuation of the senses. In The Oxford handbook of philosophy of perception, ed. M. Matthen, 567–585. Oxford: Oxford University Press.
McConwell and Currie. 2017. Gouldian arguments and the sources of contingency. Biology and Philosophy 32: 243–261.
McDowell, J. 1994. Mind and world. Cambridge, MA: Harvard University Press.
Mestre, D. R., M. Brouchon, M. Ceccaldi, and M. Poncet. 1992. Perception of optical flow in cortical blindness: A case report. Neuropsychologia 30 (9): 783–795.
Mikhalevich, I., and R. Powell. 2020. Minds without spines: Evolutionarily inclusive animal ethics. Animal Sentience, 29 (1).
Milner, A. D. 2012. Is visual processing in the dorsal stream accessible to consciousness? Proceedings of the Royal Society of London B: Biological Sciences, 279(1737), 2289–2298.
Milner, A. D., and M. A. Goodale. 2008. Two visual systems re-viewed. Neuropsychologia 46 (3): 774–785.
Müller, G. B. 2003. Homology: The evolution of morphological organization. In Origination of organismal form: Beyond the gene in developmental and evolutionary biology, eds. G. B. Müller, and S. A. Newman, 51–69. Cambridge, MA: MIT Press.
Müller, G. B. 2010. Epigenetic innovation. In Evolution: The extended synthesis, eds. M. Pigliucci, and G. B. Müller, 307–332. Cambridge, MA: MIT Press.
Naccache, L. 2006. Is she conscious? Science 313 (5792): 1395–1396.
Nelkin, N. 1995. The dissociation of phenomenal states from apperception. In Conscious experience, ed. T. Metzinger, 373–383. Thorverton, UK: Imprint Academic.
Newcomb, J. M., A. Sakurai, J. L. Lillvis, C. A. Gunaratne, and P. S. Katz. 2012. Homology and homoplasy of swimming behaviors and neural circuits in the Nudipleura (Mollusca, Gastropoda, Opisthobranchia). Proceedings of the National Academy of Sciences of the United States of America 109 (Supplement 1): 10669–10676.
Nguyen, A. P., M. L. Spetch, N. A. Crowder, I. R. Winship, P. L. Hurd, and D. R. Wylie. 2004. A dissociation of motion and spatial-pattern vision in the avian telencephalon: Implications for the evolution of “visual streams”. Journal of Neuroscience: The Official Journal of the Society for Neuroscience 24 (21): 4962–4970.
Parker, D. M., and J. D. Delius. 1980. The effects of wulst lesions on simple visual discrimination performance in the pigeon. Behavioural Processes 5 (2): 151–159.
Price, J. J., K. P. Johnson, and D. H. Clayton. 2004. The evolution of echolocation in swiftlets. Journal of Avian Biology 35 (2): 135–143.
Polger, T. 2017. Rethinking the evolution of consciousness. In The Blackwell companion to consciousness, eds. S. Schneider, and M. Velmans, 77–92 (second edn.). Oxford: Blackwell.
Powell, R. 2020. Contingency and convergence: Towards a cosmic biology of body and mind. Cambridge, MA: MIT Press.
Powell, R., and N. Shea. 2014. Homology across inheritance systems. Biology & Philosophy 29 (6): 781–806.
Ramsey, G., and A. S. Peterson. 2012. Sameness in biology. Philosophy of Science 79 (2): 255–275.
Rendall, D., and A. Di Fiore. 2007. Homoplasy, homology, and the perceived special status of behavior in evolution. Journal of Human Evolution 52 (5): 504–521.
Robinson, W. 2007. Evolution and epiphenomenalism. Journal of Consciousness Studies 14 (11): 27–42.
Robinson, Z., C. J. Maley, and G. Piccinini. 2015. Is consciousness a spandrel? Journal of the American Philosophical Association 1 (2): 365–383.
Rose, J. D. 2007. Anthropomorphism and “mental welfare”of fishes. Diseases of Aquatic Organisms 75 (2): 139–154.
Rose, J. D., R. Arlinghaus, S. J. Cooke, B. K. Diggles, W. Sawynok, E. D. Stevens, and C. D. L. Wynne. 2014. Can fish really feel pain? Fish and Fisheries 15 (1): 97–133.
Rosenthal, D. M. 1986. Two concepts of consciousness. Philosophical Studies 49 (3): 329–359.
Rosenthal, D. M. 2002. Explaining consciousness. In Philosophy of mind: Classical and contemporary readings, ed. D. J. Chalmers, 406–421. Oxford: Oxford University Press.
Rosenthal, D. M. 2008. Consciousness and its function. Neuropsychologia 46 (3): 829–840.
Roth, V. L. 1984. On homology. Biological Journal of the Linnean Society 22: 13–29.
Roth, V. L. 1991. Homology and hierarchies: Problems solved and unresolved. Journal of Evolutionary Biology 4: 167–194.
Ryle, G. 1949. The concept of mind. New York: Barnes and Noble Books.
Sakurai, A., and P. S. Katz. 2017. Artificial synaptic rewiring demonstrates that distinct neural circuit configurations underlie homologous behaviors. Current Biology 27 (12): 1721–1734.e3.
Sakurai, A., J. M. Newcomb, J. L. Lillvis, and P. S. Katz. 2011. Different roles for homologous interneurons in species exhibiting similar rhythmic behaviors. Current Biology 21 (12): 1036–1043.
Schmitz, T. W., and S. C. Johnson. 2007. Relevance to self: A brief review and framework of neural systems underlying appraisal. Neuroscience and Biobehavioral Reviews 31 (4): 585–596.
Schütt, C., and R. Nöthiger. 2000. Structure, function and evolution of sex-determining systems in Dipteran insects. Development 127 (4): 667–677.
Searle, J. R. 1992. The rediscovery of the mind. Cambridge, MA: MIT Press.
Seth, A. K. 2016. Why fish pain cannot and should not be ruled out. Animal Sentience, 3 (14).
Shimizu, T., and A. N. Bowers. 1999. Visual circuits of the avian telencephalon: Evolutionary implications. Behavioural Brain Research 98 (2): 183–191.
Shimizu, T., and S. Watanabe. 2012. The avian visual system: Overview. In How animals see the world: comparative behavior, biology, and evolution of vision, eds. O. F. Lazareva, T. Shimizu, and E. A. Wasserman, 473–482. Oxford: Oxford University Press.
Shimizu, T., T. B. Patton, and S. A. Husband. 2010. Avian visual behavior and the organization of the telencephalon. Brain, Behavior and Evolution 75 (3): 204–217.
Shubin, N., C. Tabin, and S. Carroll. 1997. Fossils, genes and the evolution of animal limbs. Nature 388: 639–648.
Shubin, N., C. Tabin, and S. Carroll. 2009. Deep homology and the origins of evolutionary novelty. Nature 457: 818–823.
Sneddon, L. U. 2003. The evidence for pain in fish: The use of morphine as an analgesic. Applied Animal Behaviour Science 83 (2): 153–162.
Sneddon, L. 2011. Pain perception in fish. Journal of Consciousness Studies 18 (9–10): 209–229.
Sneddon, L. U., R. W. Elwood, S. A. Adamo, and M. C. Leach. 2014. Defining and assessing animal pain. Animal Behaviour 97: 201–212.
Sommer, R. J. 2008. Homology and the hierarchy of biological systems. BioEssays 30 (7): 653–658.
Stanovich, K. E. 2005. The robot’s rebellion: Finding meaning in the age of Darwin. Chicago: University of Chicago Press.
Stanovich, K. E., and R. F. West. 2000. Advancing the rationality debate. Behavioral and brain sciences 23 (5): 701–717.
Striedter, G. F. 2019. Variation across species and levels: Implications for model species research. Brain, Behavior and Evolution 93 (2–3): 57–69.
Suzuki, D. G., and S. Tanaka. 2017. A phenomenological and dynamic view of homology: Homologs as persistently reproducible modules. Biological Theory 12 (3): 169–180.
Thoreson, W. B., and D. M. Dacey. 2019. Diverse cell types, circuits, and mechanisms for color vision in the vertebrate retina. Physiological Reviews 99 (3): 1527–1573.
True, J. R., and E. S. Haag. 2001. Developmental system drift and flexibility in evolutionary trajectories. Evolution and Development 3 (2): 109–119.
Tye, M. 2017. Tense bees and shell-shocked crabs: Are animals conscious? New York: Oxford University Press.
Van Gulick, R. 1989. What difference does consciousness make? Philosophical Topics 17 (1): 211–230.
Velmans, M. 2007. The co-evolution of matter and consciousness. Synthesis philosophica 22 (44): 273–282.
Wagner, G. P. 1989. The biological homology concept. Annual Review of Ecology, Evolution, and Systematics 20: 51–69.
Wagner, G. P. 2014. Homology, genes, and evolutionary innovation. Princeton: Princeton University Press.
Wakita, M., S. Watanabe, T. Shimizu, and L. R. Britto. 1992. Visual discrimination performance after lesions of the ventral lateral geniculate nucleus in pigeons (Columba livia). Behavioural Brain Research 51 (2): 211–215.
Walmsley, L. D. 2020. Lessons from a virtual slime: marginal mechanisms, minimal cognition and radical enactivism. Adaptive Behavior 28 (6): 453–464.
Weiskrantz, L., E. K. Warrington, M. D. Sanders, and J. Marshall. 1974. Visual capacity in the hemianopic field following a restricted occipital ablation. Brain 97 (4): 709–728.
Wylie, D. R., C. Gutierrez-Ibanez, J. M. Pakan, and A. N. Iwaniuk. 2009. The optic tectum of birds: Mapping our way to understanding visual processing. Canadian Journal of Experimental Psychology 63 (4): 328–338.
Young, G. 2006. Preserving the role of conscious decision making in the initiation of intentional action. Journal of Consciousness Studies 13 (3): 51–68.
Acknowledgements
We would like to thank the two anonymous referees for their very helpful comments.
Funding
This work was supported by JSPS KAKENHI Grant Number 20K00275.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The wrong Supplementary files were originally published with this article; they have been removed now.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ota, K., Suzuki, D.G. & Tanaka, S. Phylogenetic Distribution and Trajectories of Visual Consciousness: Examining Feinberg and Mallatt’s Neurobiological Naturalism. J Gen Philos Sci 53, 459–476 (2022). https://doi.org/10.1007/s10838-021-09591-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10838-021-09591-1