Abstract
We present the results of obtaining and investigating multiferroic ceramics Pb(Fe1/2Nb1/2)O3 (PFN) and PFN doped by various amounts of Li (PFN:Li). Ceramics have been obtained from oxides by two-step synthesis. For obtained samples the X-ray diffraction patterns, microstructure, DC electric conductivity, the temperature dependencies of dielectric permittivity and dielectric losses, electromechanical properties and hysteresis loops have been investigated. Obtained results have shown that the introduction of Li decreases electric conductivity and improves dielectric and electromechanical parameters important for applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Intelligent materials are able to change their properties according to external factors and those changes remain after removing the factors mentioned. Materials with constant properties which do not change properties in such a way (for example: gold, silver, platinum, diamond etc.) cannot be included into the group of intelligent materials (also called smart materials). Intelligent materials have one or more properties that can be dramatically altered. There are many types of smart materials, for example piezoelectric materials, fluids changing their viscosity under magnetic or electric field (magneto-rheostatic or electro-rheostatic materials) and shape memory alloys. The special group of smart materials are ferroics and multiferroics. Ferroics are materials in which the answer of hysteretic type is related to only one precisely defined external factor. The term multiferroic was first used by H. Schmid in 1994 [1] for materials that exhibit more than one primary ferroic order parameter simultaneously with another ones. Materials with two ferroic order parameters are called biferroics. Recently, a lot of excellent theoretical and review papers have been published for example [2–6]. Materials exhibiting such activity can be used in various forms (single crystals, polycrystallic ceramic materials, composite materials, thin films, multilayer materials etc.) for construction of many units (for example artificial intelligence units [7]). Obtaining such materials requires advanced technologies.
Pb(Fe1/2Nb1/2)O3 (PFN) belongs to the biferroic materials. Undoped PFN exhibits simultaneously ferroelectric and antiferromagnetic properties below -130°C (143 K). At the temperatures range from -130°C (143 K) to +115°C (388 K) this material is a relaxor ferroelectric. This compound has been described by Smolensky [8] and Venevcev [9] in the fifties and sixties of the previous century. The structure of PFN is perovskite type. The elementary cell contains Fe3+ ions and Nb5+ in B-positions, while Pb2+ ion occupies A-positions [10]. According to relatively easy process of synthesis, low temperatures of sintering, low reactivity and high value of dielectric permittivity, PFN is still an attractive material for commercial electroceramics [11]. The main problem during obtaining PFN ceramics is formation of the second non-perovskite phase (pyrochlore) and related to this high dielectric losses and high dielectric conductivity. It narrows down the possibility of wide applications of this material in the electronic devices. It is possible to reduce these negative phenomena by doping PFN, for example by Li [12]. Another possibility is appropriate and careful processing of ceramics with excess of PbO.
In this work we have developed the technology of PFN and PFN:Li. For the obtained samples we investigated the effects of lithium doping on the electrical conductivity and another basic parameters of PFN and PFN:Li ceramics. Li addition has been introduced into the PFN base in the amount of 0.5, 1.0, 1.5, 2.0% at.
2 Experimental
Ceramic samples of PbFe1/2Nb1/2O3 have been obtained using two-step technology (called the columbite method although the columbite is MgNbO3). The technological steps were as follows:
-
drying and weighting the powders Fe2O3 and Nb2O5 for obtaining FeNbO4 (i.e. without PbO);
-
mixing the powders for 8 h (at every stage of the technology of FeNbO4 and PFN);
-
the synthesis of powders (calcination) in the air according to reaction: Fe2O3+Nb2O5→2FeNbO4 in conditions: T s1 = 1000°C and t s1 = 4 h;
-
weighting FeNbO4, PbO and Li2CO3. Expecting the possible losses of the lead the 2.5% excess of PbO has been added. Lithium has been introduced as Li2CO3 according to reaction: \( 0.{\hbox{5FeNb}}{{\hbox{O}}_{{4}}} + \left( {{1} - x} \right){\hbox{PbO}} + x{\hbox{L}}{{\hbox{i}}_{{2}}}{\hbox{C}}{{\hbox{O}}_{{3}}} \to {\hbox{P}}{{\hbox{b}}_{{{1} - x}}}{\hbox{L}}{{\hbox{i}}_{{{2}x}}}\left( {{\hbox{F}}{{\hbox{e}}_{{{1}/{2}}}}{\hbox{N}}{{\hbox{b}}_{{{1}/{2}}}}} \right){{\hbox{O}}_{{3}}} + x{\hbox{C}}{{\hbox{O}}_{{2}}} \uparrow \) with x = 0.0025, 0.05, 0.075, 0.01. Samples obtained in such a way are denoted below PL0.5FN, PL1.0FN, PL1.5FN and PL2.0FN;
-
the synthesis (calcination) of such prepared powders at T s2 = 800°C and t s2 = 3 h;
-
pressing into pellets;
-
the final sintering in alumina crucibles in air at T s = 1050°C and t s = 2 h.
The obtained samples were discs with diameter ϕ ≈ 10 mm and thickness d ≈ 1 mm. Silver electrodes have been put on both sides of the samples.
The following investigations have been carried out for obtained ceramic samples:
-
XRD tests of crystallographic structure using Philips X’pert diffractometer
-
the microstructure investigations using SEM HITACHI S-4700 scanning microscope
-
resistivity measurements in the temperature range (20–400)°C using Tesla BM518 m
-
the measurements of dielectric parameters using QuadTech 1920 Precision LCR Meter
-
electromechanical measurements using Philtec Inc. D63 and high voltage amplifier Matsusada HEOPS-5B6 controlled by PC
-
the hysteresis loop investigations using the same equipment as for electromechanical measurements.
3 Results and discussion
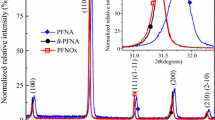
X-ray diffraction patterns for all investigated compositions are presented in Fig. 1 (in logarithmic scale). All maxima belong to the perovskite phase, so we can conclude that the samples are single phase—perovskite (i.e. without the pyrochlore phase). At the room temperature the structure of the samples is tetragonal. It has also been observed that the introduction of Li into PFN slightly changes the elementary cell parameters (Table 1), however we did not observe any distinct relation between the amount of lithium addition and the volume of elementary cell.
The obtained material has high density (Table 1) and relatively large grains with dimensions between 3.2 ÷ 5.5 μm. On the basis of the selected SEM images of the samples fractures (Fig. 2) it can also be stated that large heterogeneity of grain sizes takes place. It is seen in Fig. 2(a), (b) and (c) that all the grains are broken. On the other hand in case of Fig. 2(d) and e there are also seen grains which are unbroken what can be related to the fact that the strength of grain inside is higher than at the grain boundaries. So we can conclude that the introduction of lithium at the amount 1.5%at. and 2%at. improves the strength of grain boundaries. The optimal shape of grains which is seen in Fig. 2(e) i.e. for PLi2.0FN is connected with the highest strength of grain boundaries as well as the insides.
Figure 3 shows the dependency lnσ(1/T) for PFN and PFN:Li. For lithium amount below 1.5%at. with increasing Li contents DC conductivity decreases. For the linear parts of lnσ(1/T) plots it was possible to calculate the activation energy from formula:
where: σ—conductivity at given temperature, σ 0 - pre-exponential factor, E a - activation energy, k - Boltzman constant, T - temperature. Values of activation energy obtained in such a way (Table 1) are of the same order as for example in works [12, 13]. At the low temperatures (range I) the values of activation energy are lower than the values at the highest temperatures (range III). There are also the samples in which it was possible to distinguish three temperature ranges. Generally the higher the Li amount the higher the activation energy (Table 1). In the work of Zieleniec et. al. [12] the influence of the Li addition on the type of conductivity of PFN ceramics was also investigated. It has been stated that for Li amount smaller than 0.5%at. the conductivity is n-type within the temperature range 102 ÷ 452°C (375 ÷ 725 K) though the values of activation energy change in this range. For 1%at. Li the conductivity is p-type. For 2%at. Li the conductivity is change at about 297°C (570 K) from p-type to n-type (on heating).
The influence of Li addition into PFN ceramics on lnσ (1/T) dependencies I, II, III—indicates ranges with different activation energy, respectively E a1 , E a2 , E a3 —see Table 1
Dependencies ε(T) measured at 1 kHz are presented in Fig. 4. It is seen that with increasing Li content the maximum of dielectric permittivity increases with simultaneous shift towards lower temperatures. The highest temperature of the phase transition was observed for undoped PFN, while the lowest one for PL2.0FN (Fig. 4 and Table 1).
Figure 4 also shows that for all samples the phase transition is diffused although the degree of diffusion is different. As a measure of the phase transition diffusion the ratio between the maximum dielectric permittivity (at temperature T m ) and the dielectric permittivity at the room temperature (ε m /ε r ) (see Table 1) can be taken. The degree of diffusion can be also calculated (only for paraelectric phase) from the formula:
where: T m —the temperature at which dielectric permittivity riches its maximum (ε m ), ε m —dielectric permittivity at the temperature T m , C—temperature independent constant, α—exponent related with the degree of diffusion of the phase transition. After finding the logarithm we obtain:
At the case α = 1 and ε m = 1 we obtain normal Curie-Weiss law, while for 1 < α ≤2 the phase transitions is diffused. Figure 5 presents the plots ln(1/ε-1/ε m ) vs. ln(T-T m ) for T > T m . Fitting data to Eq. 4 we can calculate the α parameter values. Obtained results are presented in Table 1. It is seen that with increasing Li addition the diffusion of the phase transition becomes less diffused (α decreases from 1.93 for undoped PFN to 1.74 for 2%Li).
In Fig. 6 we compared the temperature dependencies of dielectric losses (tanδ) measured with the frequency 1 kHz. For undoped PFN the value of tanδ changes only in minimal degree between the room temperature and temperature about 115 C. Above temperature of phase transition tanδ increases abruptly, which is probably the result of a conductivity increase. In samples doped by Li the anomalies of tanδ are observed at temperatures below the phase transition temperature. The highest values of tanδ have been observed for undoped PFN (Table 1).
The results of P-E hysteresis loops investigations (i.e. polarization switching) at room temperature and the frequency 1 Hz are shown in Fig. 7. A distinct difference in the shape of hysteresis loop between undoped PFN and Li-doped samples can be observed. The loops for PFN:Li are more saturated than undoped PFN. With increasing Li-content the value of spontaneous polarization (P s )also and remanent polarization (P r ) increase while the coercion field (E C ) decreases.
In Fig. 8 S-E (strain-electric field) loops for investigated samples are presented. It is seen that with increasing Li-content, the shape of S-E loops changes from that typical for relaxors to that typical for normal ferroelectrics. For describing of S-E loops shape we can use coefficient of strain H s and the remanent strain S r [%]. Coefficient H s can be defined as:
where: ΔS half —the hysteresis of strains (i.e. the difference between maximum and minimum strain for the half of the maximum electric field [%], S max —strain for the maximum electric field [%]. The obtained values are presented in Table 1. The maximum value of remanent strain S r has been obtained for PL2.0FN.
4 Conclusions
Above we have presented the results of investigations of PFN and PFN:Li ceramics obtained by two-step columbite method with Li-addition in amounts 0.5% at., 1.0% at.,1.5% at. and 2.0% at.
The microstructure studies have shown that the addition of lithium significantly influences the grain’s sizes, shapes and properties. The best microstructure is for 2.0%at. Li.
XRD investigations have shown that the samples are single phase—perovskite (i.e. without the pyrochlore phase).
Li addition decreases the diffusion of phase transition and increases the maximum value of dielectric permittivity. The maximum of dielectric permittivity shifts towards lower temperatures. The Li-addition influences the shape of P-E and S-E hysteresis loops. With increasing lithium amount both these loops become more typical for ferroelectric materials. Other properties also become similar to normal ferroelectrics.
As a result we conclude that Li-doped PFN ceramics will be interesting materials for applications in electronic devices.
References
H. Schmid, Ferroelectrics 162, 317 (1994)
S. Picozzi et al., J Phys Condens Matter 20(43), 434208 (2008)
H. Schmid, J Phys Condens Matter 20(43), 434201 (2008)
W. Eerenstein, N.D. Mathur, J.F. Scott, Nature 442, 759 (2006)
S.W. Cheong, M. Mostovoy, Multiferroics: a magnetic twist for ferroelectricity. Nat Mater 6, 13 (2007)
Z. Surowiak, D. Bochenek, J. Korzekwa, Electron Telecommunications Q 53(2), 193 (2007)
Z. Surowiak, D. Bochenek, Archives of Acoustic 33(2), 243 (2008)
G.A. Smoleński, W.M. Judin, Fiz. Twierdogo Tela 6, 3668 (1964)
Y.E. Roginskaya, Y.N. Venevcev, S.A. Fedulov, Sov. Phys. Crystallogr. 8, 490 (1964)
V.V. Bhat, K.V. Ramanujachary, S.E. Lofland, A.M. Umarli, J Magn Magn Mater 280, 221 (2004)
J. Tand, M. Zhu, T. Hong, Y. Hou, H. Wang, H. Yan, Mater Chem Phys 101, 475 (2007)
K. Wójcik, K. Zieleniec, M. Mulata, Ferroelectrics 289, 107 (2003)
O. Raymond, R. Font, N. Juarez-Almodovar, J. Portelies, J.M. Siqueiros, J Appl Phys 97(084107), 1–8 (2005)
Acknowledgements
Work supported by Polish Grant N N507 480237.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Bochenek, D., Kruk, P., Skulski, R. et al. Multiferroic ceramics Pb(Fe1/2Nb1/2)O3 doped by Li. J Electroceram 26, 8–13 (2011). https://doi.org/10.1007/s10832-010-9620-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10832-010-9620-9