Abstract
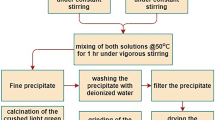
Mono-disperse and spherical Ni powders were prepared using a N2H4-based solution reduction route. The main focus was on manipulating the particle size by varying the source materials (Ni-chloride, Ni-sulfate and Ni-acetate) and impurity concentrations. The morphology and size of the Ni particle closely depended on the source materials. In addition, the particle size became significantly smaller with increasing Co concentration ranging from 0 to 350 ppm. The decrease in the Ni particle size was attributed to the promotion of nucleation by the rapid decomposition of the CoCl2–N2H4 complex when adding a NaOH solution and the more active reduction of Co than that of Ni in the initial stages of the reaction.
Similar content being viewed by others
References
H. Kishi, Y. Mizuno, and H. Chaozono, Jpn. J. Appl. Phys., 42, 1 (2003).
J.-Y. Lee, J.-H. Lee, S.-H. Hong, Y.K. Lee, and J.-Y. Choi, Adv. Mater., 15, 1655 (2003).
J.-H. Hwang, V.P. Dravid, M.H. Teng, J.J. Host, B.R. Elliott, D. L. Johnson, and T.O. Mason, J. Mater. Res., 12(4), 1076 (1997).
S. Stopić, J. Nedeljković, S. Rakoçević, and D. Uskoković, J. Mater. Res., 14, 3059 (1999).
B. Xia, I.W. Lenggoro, and K. Okuyama, J. Mater. Res., 15, 2157 (2000).
F. Fiévet, J.P. Lagier, and M. Figlarz, MRS. Bull., 14, 29 (1989).
V. Viau, F. Fiévet-Vincent, and F. Fiévet, Solid State Ionics, 84, 259 (1996).
D.-H. Chen and S.-H. Wu, Chem. Mater., 12, 1354 (2000).
Y.D. Li, C.W. Li, H.R. Wang, L.Q. Li, and Y.T. Qian, Mater. Chem. Phys., 59, 88 (1999).
Z. Gui, R. Fan, W. Mo, X. Chen, L. Yang, and Y. Hu, Mater. Res. Bull., 38, 169 (2003).
J. Gao, F. Guan, Y. Zhao, W. Yang, Y. Ma, X. Lu, J. Hou, and J. Kang, Mater. Sci. Commun., 71, 215 (2001).
R.S. Sapieszko and E. Matijeviç, Corrosion-Nace, 36, 522 (1980).
A. Degan and J. Maçek, Nanostructured Mater., 12, 225 (1999).
J.Y. Choi, Y.-K. Lee, S.-M. Yoon, H.C. Lee, B.-K. Kim, J.M. Kim, K.-M. Kim, and J.-H. Lee, J. Am. Ceram. Soc., 88, 3020 (2005).
A.J. Bard and L.R. Faulkner, Electrochemical methods fundamentals and applications. John Wiley & Sons, NY (1980).
F. Guo, H. Zheng, Z. Yang, and Y. Qian, Mater. Lett., 56, 906 (2002).
J. McMurry and Robert C. Fay, Chemistry, 4th ed., Prentice-Hall, NJ (2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, KM., Lee, JH., Yoon, SM. et al. Preparation of mono-disperse Ni powders via the reduction of hydrazine complexes: The effect of source materials and impurities. J Electroceram 17, 339–343 (2006). https://doi.org/10.1007/s10832-006-9117-8
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10832-006-9117-8