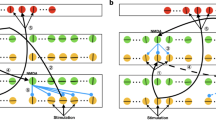
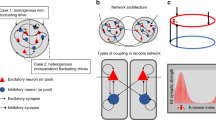
Abstract
Binocular rivalry occurs when two very different images are presented to the two eyes, but a subject perceives only one image at a given time. A number of computational models for binocular rivalry have been proposed; most can be categorised as either “rate” models, containing a small number of variables, or as more biophysically-realistic “spiking neuron” models. However, a principled derivation of a reduced model from a spiking model is lacking. We present two such derivations, one heuristic and a second using recently-developed data-mining techniques to extract a small number of “macroscopic” variables from the results of a spiking neuron model simulation. We also consider bifurcations that can occur as parameters are varied, and the role of noise in such systems. Our methods are applicable to a number of other models of interest.

















Similar content being viewed by others
References
Ashwin, P., & Lavric, A. (2010). A low-dimensional model of binocular rivalry using winnerless competition. Physica D (in press).
Bahraminasab, A., Kenwright, D., Stefanovska, A., Ghasemi, F., & McClintock, P. (2008). Phase coupling in the cardiorespiratory interaction. IET Systems Biology, 2, 48–54.
Bengio, Y., Delalleau, O., Le Roux, N., Paiement, J., Vincent, P., & Ouimet, M. (2004). Learning eigenfunctions links spectral embedding and kernel PCA. Neural Computation, 16(10), 2197–2219.
Blake, R. (2001). A primer on binocular rivalry, including current controversies. Brain and Mind, 2(1), 5–38.
Blake, R., & Logothetis. N., (2002) Visual competition. Nature Reviews Neuroscience, 3(1), 1–11.
Cai, D., Tao, L., Shelley, M., & McLaughlin, D. (2004). An effective kinetic representation of fluctuation-driven neuronal networks with application to simple and complex cells in visual cortex. Proceedings of the National Academy of Sciences of the United States of America, 101(20), 7757.
Coifman, R., & Lafon, S. (2006). Diffusion maps. Applied and Computational Harmonic Analysis, 21(1), 5–30.
Dayan, P. (1998). A hierarchical model of binocular rivalry. Neural Computation, 10(5), 1119–1135.
Erban, R., Frewen, T., Wang, X., Elston, T., Coifman, R., Nadler, B., et al. (2007). Variable-free exploration of stochastic models: A gene regulatory network example. The Journal of Chemical Physics, 126, 155,103.
Ermentrout, B. (1994), Reduction of conductance-based models with slow synapses to neural nets. Neural Computation, 6(4), 679–695.
Freeman, A. (2005). Multistage model for binocular rivalry. Journal of Neurophysiology, 94(6), 4412–4420.
Friedrich, R., Siegert, S., Peinke, J., Lück, S., Siefert, M., Lindemann, M., et al. (2000). Extracting model equations from experimental data. Physics Letters A, 271(3), 217–222.
Gerstner, W., & Kistler, W. (2002). Spiking neuron models: An introduction. New York: Cambridge University Press.
Gradišek, J., Siegert, S., Friedrich, R., & Grabec, I. (2000). Analysis of time series from stochastic processes. Physical Review E, 62(3), 3146–3155.
Grossberg, S., Yazdanbakhsh, A., Cao, Y., & Swaminathan, G. (2008). How does binocular rivalry emerge from cortical mechanisms of 3-D vision? Vision Research, 48(21), 2232–2250.
Gutkin, B., Laing, C., Colby, C., Chow, C., & Ermentrout, G. (2001). Turning on and off with excitation: The role of spike-timing asynchrony and synchrony in sustained neural activity. Journal of Computational Neuroscience, 11(2), 121–134.
Jolliffe, I. (2002). Principal component analysis. New York: Springer
Jolly, M., Kevrekidis, I., & Titi, E. (1990). Approximate inertial manifolds for the Kuramoto–Sivashinsky equation: Analysis and computations. Physica D, 44(1–2), 38–60.
Kalarickal, G., & Marshall, J. (2000). Neural model of temporal and stochastic properties of binocular rivalry. Neurocomputing, 32–33, 843–853.
Kevrekidis, I., Gear, C., Hyman, J., Kevrekidis, P., Runborg, O., & Theodoropoulos, C. (2003). Equation-free, coarse-grained multiscale computation: Enabling microscopic simulators to perform system-level analysis. Communications in Mathematical Sciences, 1(4), 715–762.
Kuusela, T., Shepherd, T., & Hietarinta, J. (2003). Stochastic model for heart-rate fluctuations. Physical Review E, 67(6), 061,904.
Lago-Fernandez, L., & Deco, G. (2002). A model of binocular rivalry based on competition in IT. Neurocomputing, 44–46, 503–507.
Laing, C. (2006). On the application of “equation-free” modelling to neural systems. Journal of Computational Neuroscience, 20(1), 5–23.
Laing, C., & Chow, C. (2001). Stationary bumps in networks of spiking neurons. Neural Computation, 13(7), 1473–1494.
Laing, C., & Chow, C. (2002). A spiking neuron model for binocular rivalry. Journal of Computational Neuroscience, 12(1), 39–53.
Laing, C., Frewen, T., & Kevrekidis, I. (2007). Coarse-grained dynamics of an activity bump in a neural field model. Nonlinearity, 20(9), 2127–2146.
Leopold, D., & Logothetis, N. (1999). Multistable phenomena: Changing views in perception. Trends in Cognitive Sciences, 3(7), 254–264.
Logothetis, N., Leopold, D., & Sheinberg, D. (1996) What is rivalling during binocular rivalry? Nature, 380(6575), 621–624.
Makeev, A., Maroudas, D., & Kevrekidis, I. (2002). “Coarse” stability and bifurcation analysis using stochastic simulators: Kinetic Monte Carlo examples. The Journal of Chemical Physics, 116, 10,083.
Marder, E., & Bucher, D. (2001). Central pattern generators and the control of rhythmic movements. Current Biology, 11(23), 986–996.
Moreno-Bote, R., Rinzel, J., & Rubin, N. (2007). Noise-induced alternations in an attractor network model of perceptual bistability. Journal of Neurophysiology, 98(3), 1125.
van Mourik, A., Daffertshofer, A., & Beek, P. (2006). Deterministic and stochastic features of rhythmic human movement. Biological Cybernetics, 94(3), 233–244.
Nadler, B., Lafon, S., Coifman, R., & Kevrekidis, I. (2006). Diffusion maps, spectral clustering and reaction coordinates of dynamical systems. Applied and Computational Harmonic Analysis, 21, 113–127.
Ragwitz, M., & Kantz, H. (2001). Indispensable finite time corrections for Fokker–Planck equations from time series data. Physical Review Letters, 87(25), 254,501.
Rega, G., & Troger, H. (2005). Dimension reduction of dynamical Systems: Methods, models, applications. Nonlinear Dynamics, 41(1), 1–15.
Rybak, I., Shevtsova, N., Paton, J., Dick, T., St-John, W., Mörschel, M., et al. (2004). Modeling the ponto-medullary respiratory network. Respiratory Physiology & Neurobiology, 143(2–3), 307–319.
Shpiro, A., Moreno-Bote, R., Rubin, N., & Rinzel, J. (2009). Balance between noise and adaptation in competition models of perceptual bistability. Journal of Computational Neuroscience, 27(1), 37–54.
Shriki, O., Hansel, D., & Sompolinsky, H. (2003). Rate models for conductance-based cortical neuronal networks. Neural Computation, 15(8), 1809–1841.
Stollenwerk, L., & Bode, M. (2003). Lateral neural model of binocular rivalry. Neural Computation, 15(12), 2863–2882.
Tong, F., Meng, M., & Blake, R. (2006). Neural bases of binocular rivalry. Trends in Cognitive Sciences, 10(11), 502–511.
Tranchina, D. (2009). Population density methods in large-scale neural network modelling. In C. Laing, & G. J. Lord (eds) Stochastic methods in neuroscience (pp. 181–216). Oxford: Oxford University Press.
Wilson, H. (2003). Computational evidence for a rivalry hierarchy in vision. Proceedings of the National Academy of Sciences, USA, 100, 14,499–14,503.
Wilson, H., & Cowan, J. (1972). Excitatory and inhibitory interactions in localized populations of model neurons. Biophysical Journal, 12(1), 1–24.
Acknowledgements
The work of C.R.L. was partially supported by the Marsden Fund, administered by The Royal Society of New Zealand. The work of I.G.K. and T.F. was partially supported by the National Science Foundation and DARPA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: Carson C. Chow
Appendix: A model equations
Appendix: A model equations
Here we present the model equations. They are very similar to those in Laing and Chow (2002). For each excitatory neuron we have
where \(I_{mem}(V_e,n_e,h_e)=g_L(Ve-V_L)+g_Kn_e^4(V_e-V_K)+g_{Na}(m_{\infty}(V_e))^3h_e(V_e-V_{Na})\) and I AHP = g AHP [Ca]/([Ca] + 1)(V e − V K ). Other functions are m ∞ (V) = α m (V)/(α m (V) + β m (V)), α m (V) = 0.1(V + 30)/ \((1-\exp{[-0.1(V+30)]})\), \(\beta_{m}(V)=4\exp[-(V+55)/\) 18], \(\alpha_n(V)=0.01(V+34)/(1-\exp{[-0.1(V+34)]})\), \(\beta_n(V)=0.125\exp{[-(V+44)/80]}\), \(\alpha_h(V)=0.07\exp\) [ − (V + 44)/20], \(\beta_h(V)=1/(1+\exp{[-0.1(V+14)]})\), \(\sigma(V)=1/(1+\exp{[-(V+20)/4]}\). Parameters are g L = 0.05 mS/cm2, V L = − 65 mV, g K = 40 mS/cm2, V K = − 80 mV, g Na = 100 mS/cm2, V Na = 55 mV, V Ca = 120 mV, g AHP = 0.05 mS/cm2, g Ca = 0.1 mS/cm2, ψ = 3, τ e = 8 ms, \(\tau_g=\text{1,000}\) ms and A = 20. B is initially 1.3, but is varied in Section 4.
For each inhibitory neuron we have
where τ i = 10 ms and other functions are as above.
The synaptic current entering the jth excitatory neuron is
where \(V_e^j\) is the voltage of the jth excitatory neuron in mV, \(s_{e/i}^k\) is the strength of the synapse emanating from the kth excitatory/inhibitory neuron, ϕ k is the factor by which the kth excitatory neuron is depressed, N = 60 is the number of excitatory neurons (equal to the number of inhibitory neurons), and the Gaussian coupling functions are given by
and
The reversal potentials are V + = 0 mV, V − = − 80 mV. Similarly, the synaptic current entering the jth inhibitory neuron is
where \(V_i^j\) is the voltage of the jth inhibitory neuron in mV, and the coupling functions are
and
Parameters are α ee = 0.285 mS/cm2, α ie = 0.36 mS/cm2, α ei = 0.2 mS/cm2 and α ii = 0.07 mS/cm2. The external current to the jth excitatory neuron in μA/cm2 is
i.e. the external current injected into the excitatory population consists of two Gaussians, centered at 1/4 and 3/4 of the way around the domain. The equations were simulated using Euler’s method with a fixed time-step of 0.02 ms, and no significant changes in the network behaviour were observed when time-steps of 0.01 or 0.005 ms were used.
Note that the system is completely deterministic. Section 5 discusses the results from simulating a stochastic version of this network.
Rights and permissions
About this article
Cite this article
Laing, C.R., Frewen, T. & Kevrekidis, I.G. Reduced models for binocular rivalry. J Comput Neurosci 28, 459–476 (2010). https://doi.org/10.1007/s10827-010-0227-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-010-0227-6