Abstract
How spiking neurons cooperate to control behavioral processes is a fundamental problem in computational neuroscience. Such cooperative dynamics are required during visual perception when spatially distributed image fragments are grouped into emergent boundary contours. Perceptual grouping is a challenge for spiking cells because its properties of collinear facilitation and analog sensitivity occur in response to binary spikes with irregular timing across many interacting cells. Some models have demonstrated spiking dynamics in recurrent laminar neocortical circuits, but not how perceptual grouping occurs. Other models have analyzed the fast speed of certain percepts in terms of a single feedforward sweep of activity, but cannot explain other percepts, such as illusory contours, wherein perceptual ambiguity can take hundreds of milliseconds to resolve by integrating multiple spikes over time. The current model reconciles fast feedforward with slower feedback processing, and binary spikes with analog network-level properties, in a laminar cortical network of spiking cells whose emergent properties quantitatively simulate parametric data from neurophysiological experiments, including the formation of illusory contours; the structure of non-classical visual receptive fields; and self-synchronizing gamma oscillations. These laminar dynamics shed new light on how the brain resolves local informational ambiguities through the use of properly designed nonlinear feedback spiking networks which run as fast as they can, given the amount of uncertainty in the data that they process.












Similar content being viewed by others
References
Amir, Y., Harel, M., & Malach, R. (1993). Cortical hierarchy reflected in the organization of intrinsic connections in macaque monkey visual cortex. Journal of Comparative Neurology, 334, 19–46.
Anzai, A., Peng, X., & Van Essen, D. C. (2007). Neurons in monkey visual area V2 encode combinations of orientations. Nature Neuroscience, 10, 1313–1321.
Bar, M., Kassam, K. S., Ghuman, A. S., Boshyan, J., Schmidt, A. M., Dale, A. M., et al. (2006). Top-down facilitation of visual recognition. Proceedings of the National Academy of Sciences, 103, 449–454.
Berke, J. D., Hetrick, V., Breck, J., & Green, R. W. (2008). Transient 23- to 30-Hz oscillations in mouse hippocampus during exploration of novel environments. Hippocampus, 18, 519–529.
Blasdel, G. G., & Lund, J. S. (1983). Termination of afferent axons in macaque striate cortex. Journal of Neuroscience, 3, 1389–1413.
Bosking, W. H., Zhang, Y., Schofield, B., & Fitzpatrick, D. (1997). Orientation selectivity and the arrangement of horizontal connections in the tree shrew striate cortex. Journal of Neuroscience, 17, 2112–2127.
Boudreau, C. E., Williford, T. H., & Maunsell, H. R. (2006). Effects of task difficulty and target likelihood in area V4 of macaque monkeys. Journal of Neurophysiology, 96, 2377–2387.
Bringuier, V., Chavane, F., Glaeser, L., & Fregnac, Y. (1999). Horizontal propagation of visual activity in the synaptic integration field of area 17 neurons. Science, 283, 695–699.
Buffalo, E. A., Fries, P., & Desimone, R. (2004). Layer-specific attentional modulation in early visual areas. Program No. 717.6. 2004 Neuroscience Meeting Planner. San Diego: Society for Neuroscience. Online. Society for Neuroscience Abstracts, 30, 717–716.
Bullier, J., Hupé, J. M., James, A., & Girard, P. (1996). Functional interactions between areas V1 and V2 in the monkey. Journal of Physiology - Paris, 90, 217–220.
Buschman, T. J., & Miller, E. K. (2008). Covert shifts in attention by frontal eye fields are synchronized to population oscillations. Submitted for publication.
Callaway, E. M., & Wiser, A. K. (1996). Contributions of individual layer 2–5 spiny neurons to local circuits in macaque primary visual cortex. Visual Neuroscience, 13, 907–922.
Cao, Y., & Grossberg, S. (2005). A laminar cortical model of stereopsis and 3D surface perception: closure and da Vinci stereopsis. Spatial Vision, 18, 515–578.
Carpenter, G. A. (1979). Bursting phenomena in excitable membranes. SIAM Journal of Applied Mathematics, 36, 334–372.
Carpenter, G., & Grossberg, S. (1993). Normal and amnesic learning, recognition, and memory by a neural model of cortico-hippocampal interactions. Trends in Neurosciences, 16, 131–137.
Chisum, H. J., Mooser, F., & Fitzpatrick, D. (2003). Emergent properties of layer 2/3 neurons reflect the collinear arrangement of horizontal connections in tree shrew visual cortex. Journal of Neuroscience, 23, 2947–2960.
Cohen, M. A., & Grossberg, S. (1984). Neural dynamics of brightness perception: features, boundaries, diffusion, and resonance. Perception & Psychophysics, 36, 428–456.
Crook, J. M., Engelmann, R., & Löwel, S. (2002). GABA-inactivation attenuates collinear facilitation in cat primary visual cortex. Experimental Brain Research, 143, 295–302.
Domijan, D., Šetic, M., & Švegar, D. (2007). A model of illusory contour formation based on dendritic computation. Neurocomputing, 70, 1977–1982.
Eckhorn, R., Bauer, R., Jordan, W., Brosch, M., Kruse, W., Munk, M., et al. (1988). Coherent oscillations: a mechanism of feature linking in the visual cortex? Biological Cybernetics, 60, 121–130.
Elston, G. N., Rosa, G. P., & Calford, M. B. (1996). Comparison of dendritic fields of Layer III pyramidal neurons in striate and extrastriate visual areas of the marmoset: a lucifer yellow intracellular injection study. Cerebral Cortex, 6, 807–813.
Fang, L., & Grossberg, S. (2009). From stereogram to surface: how the brain sees the world in depth. Spatial Vision, 22, 45–82.
Ferster, D., Chung, S., & Wheat, H. (1996). Orientation selectivity of thalamic input to simple cells of cat visual cortex. Nature, 380, 249–252.
Field, D. J., Hayes, A., & Hess, R. F. (1993). Contour integration by the human visual system—evidence for a local association field. Vision Research, 33, 173–193.
Fitzhugh, R. (1955). Mathematical models of threshold phenomena in the nerve membrane. Bulletin of Mathematical Biology, 17, 252–278.
Fitzpatrick, D. (1996). The functional organization of local circuits in visual cortex: insights from the study of tree shrew striate cortex. Cerebral Cortex, 6, 329–341.
Francis, G., Grossberg, S., & Mingolla, E. (1994). Cortical dynamics of feature binding and reset: control of visual persistence. Vision Research, 34, 1089–1104.
Fries, P., Reynolds, J. H., Rorie, A. E., & Desimone, R. (2001). Modulation of oscillatory neuronal synchronization by selective visual attention. Science, 291, 1560–1563.
Fukuda, T., Kosaka, T., Singer, W., & Galuske, R. A. W. (2006). Gap junctions among dendrites of cortical GABAergic neurons establish a dense and widespread intercolumnar network. Journal of Neuroscience, 26, 3434–3443.
Gautrais, J., & Thorpe, S. (1998). Rate coding versus temporal order coding: a theoretical approach. Biosystems, 48, 57–65.
Gilbert, C. D., & Wiesel, T. N. (1983). Clustered intrinsic connections in cat visual cortex. Journal of Neuroscience, 3, 1116–1133.
Girard, P., Hupé, J. M., & Bullier, J. (2001). Feedforward and feedback connections between areas V1 and V2 of the monkey have similar rapid conduction velocities. Journal of Neurophysiology, 85, 1328–1331.
Golubitsky, M., & Stewart, I. (2006). Nonlinear dynamics of networks: the groupoid formalism. Bulletin of the American Mathematical Society, 43, 305–364.
Gray, C. M., & Singer, W. (1989). Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proceedings of the National Academy of Sciences USA, 86, 1698–1702.
Gray, C. M., König, P., Engel, A. K., & Singer, W. (1989). Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature, 338, 334–337.
Gregoriou, G. G., Gotts, S. J., Zhou, H., & Desimone, R. (2009). High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science, 324, 1207–1210.
Grossberg, S. (1973). Contour enhancement, short term memory, and constancies in reverberating neural networks. Studies in Applied Mathematics, 52, 217–257. Reprinted in Studies of Mind and Brain, S. Grossberg (1982). D. Reidel Publishing Company: Dordrecht, Holland.
Grossberg, S. (1976a). Adaptive pattern classification and universal recoding, I: parallel development and coding of neural feature detectors. Biological Cybernetics, 23, 121–134.
Grossberg, S. (1976b). Adaptive pattern classification and universal recoding, II: feedback, expectation, olfaction, and illusions. Biological Cybernetics, 23, 187–202.
Grossberg, S. (1980). How does a brain build a cognitive code? Psychological Review, 87, 1–51.
Grossberg, S. (1984). Outline of a theory of brightness, color, and form perception. In E. Degreef & J. van Buggenhaut (Eds.), Trends in mathematical psychology (pp. 59–85). Amsterdam: North-Holland.
Grossberg, S. (1999). How does the cerebral cortex work? Learning, attention, and grouping by the laminar circuits of visual cortex. Spatial Vision, 12, 163–185.
Grossberg, S. (2003). How does the cerebral cortex work? Development, learning, attention, and 3D vision by laminar circuits of visual cortex. Behavioral and Cognitive Neuroscience Reviews, 2, 47–76.
Grossberg, S. (2007). Consciousness CLEARS the mind. Neural Networks, 20, 1040–1053.
Grossberg, S. (2009). Beta oscillations and hippocampal place cell learning during exploration of novel environments. Hippocampus, 19, 881–885.
Grossberg, S., & Grunewald, A. (1997). Cortical synchronization and perceptual framing. Journal of Cognitive Neuroscience, 9, 117–132.
Grossberg, S., & Mingolla, E. (1985a). Neural dynamics of perceptual grouping: textures, boundaries, and emergent segmentations. Perception & Psychophysics, 38, 141–171.
Grossberg, S., & Mingolla, E. (1985b). Neural dynamics of form perception: boundary completion, illusory figures, and neon color spreading. Psychological Review, 92, 173–211.
Grossberg, S., & Raizada, R. D. S. (2000). Contrast-sensitive perceptual grouping and object-based attention in the laminar circuits of primary visual cortex. Vision Research, 40, 1413–1432.
Grossberg, S., & Somers, D. (1991). Synchronized oscillations during cooperative feature linking in a cortical model of visual perception. Neural Networks, 4, 453–466.
Grossberg, S., & Swaminathan, G. (2004). A laminar cortical model of 3D perception of slanted and curved surfaces and of 2D images: development, attention and bistability. Vision Research, 44, 1147–1187.
Grossberg, S., & Versace, M. (2008). Spikes, synchrony, and attentive learning by laminar thalamocortical circuits. Brain Research, 1218, 278–312.
Grossberg, S., & Williamson, J. R. (2001). A neural model of how horizontal and interlaminar connections of visual cortex develop into adult circuits that carry out perceptual grouping and learning. Cerebral Cortex, 11, 37–58.
Grossberg, S., & Yazdanbakhsh, A. (2005). Laminar cortical dynamics of 3D surface perception: stratification, transparency, and neon color spreading. Vision Research, 45, 1725–1743.
Grossberg, S., Mingolla, E., & Ross, W. D. (1997). Visual brain and visual perception: how does the cortex do perceptual grouping? Trends in Neuroscience, 20, 106–111.
Grossberg, S., Yazdanbakhsh, A., Cao, Y., & Swaminathan, G. (2008). How does binocular rivalry emerge from cortical mechanisms of 3-D vision? Vision Research, 48, 2232–2250.
Guttman, S., Sekuler, A., & Kellman, P. J. (2003). Temporal variations in visual completion: a reflection of spatial limits? Journal of Experimental Psychology: Human Perception and Performance, 29, 1211–1227.
Halgren, E., Mendola, J., Chong, C. D. R., & Dale, A. M. (2003). Cortical activation to illusory shapes as measured with magnetoencephalography. Neuroimage, 18, 1001–1009.
Heeger, D. J. (1992). Normalization of cell responses in cat striate cortex. Visual Neuroscience, 9, 181–197.
Hirsch, J. A., & Gilbert, C. D. (1991). Synaptic physiology of horizontal connections in the cat’s visual cortex. Journal of Neuroscience, 11, 1800–1809.
Hochstein, S., & Ahissar, M. (2002). View from the top: hierarchies and reverse hierarchies in the visual system. Neuron, 36, 791–804.
Hodgkin, A. L., & Huxley, A. F. (1952). A quantitative description of membrane current and its application to conduction and excitation in nerve. Journal of Physiology, 117, 500–544.
Holmgren, C., Harkany, T., Svennenfors, B., & Zilberter, Y. (2003). Pyramidal cell communication within local networks in layer 2/3 of rat neocortex. Journal of Physiology, 551, 139–153.
Hupé, J.-M., James, A. C., Girard, P., Lomber, S. G., Payne, B. R., & Bullier, J. (2001). Feedback connections act on the early part of the responses in monkey visual cortex. Journal of Neurophysiology, 85, 134–145.
Izhikevich, E. (2007). Dynamical systems in neuroscience. Cambridge: MIT.
Kapadia, M., Westheimer, G., & Gilbert, C. D. (2000). Spatial distribution of contextual interactions in primary visual cortex and in visual perception. Journal of Neurophysiology, 84, 2048–2062.
Kellman, P. J., & Shipley, T. F. (1991). A theory of visual interpolation in object perception. Cognitive Psychology, 23, 141–221.
Kisvárday, Z. F., Tóth, É., Rausch, M., & Eysel, U. T. (1997). Orientation-specific relationship between populations of excitatory and inhibitory lateral connections in the visual cortex of the cat. Cerebral Cortex, 7, 605–618.
Koch, C. (1999). Biophysics of computation: information processing in single neurons. New York: Oxford University Press.
Köhn, J., & Wörgötter, F. (1998). Employing the Z-transform to optimize the calculation of the synaptic conductance of NMDA and other synaptic channels in network simulations. Neural Computation, 10, 1639–1651.
Lamme, V. A. F., & Roelfsema, P. R. (2000). The distinct modes of vision offered by feedforward and recurrent processing. Trends in Neuroscience, 23, 571–579.
Lamme, V. A. F., Rodriguez-Rodriguez, V., & Spekreijse, H. (1999). Separate processing dynamics for texture elements, boundaries and surfaces in primary visual cortex of the macaque monkey. Cerebral Cortex, 9, 406–413.
Lesher, G. W., & Mingolla, E. (1993). The role of edges and line-ends in illusory contour formation. Vision Research, 33, 2253–2270.
Levitt, J. B., Yoshioka, T., & Lund, J. S. (1994). Intrinsic cortical connections in macaque visual area V2: evidence for interaction between different functional streams. Journal of Comparative Neurology, 342, 551–570.
Li, Z. (1998). A neural model of contour integration in the primary visual cortex. Neural Computation, 10, 903–940.
Lund, J. S., Griffiths, S., Rumberger, A., & Levitt, J. B. (2001). Inhibitory synapse cover on the somata of excitatory neurons in macaque monkey visual cortex. Cerebral Cortex, 11, 783–795.
Lund, J. S., Angelucci, A., & Bressloff, P. C. (2003). Anatomical substrates for functional columns in macaque monkey primary visual cortex. Cerebral Cortex, 12, 15–24.
McGuire, B. A., Gilbert, C. D., Rivlin, P. K., & Wiesel, T. N. (1991). Targets of horizontal connections in macaque primary visual cortex. Journal of Comparative Neurology, 305, 370–392.
Megías, M., Emri, Z., Freund, T. F., & Gulyás, A. I. (2001). Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience, 102, 527–540.
Meyer, G., & Ming, C. (1988). The visible persistence of illusory contours. Canadian Journal of Psychology, 42, 479–488.
Milner, P. A. (1974). A model for visual shape recognition. Psychological Review, 81, 5221–5525.
Murray, R. F., Sekuler, A. B., & Bennett, P. J. (2001). Time course of amodal completion revealed by a shape discrimination task. Psychonomics Bulletin & Review, 8, 713–720.
Murray, M. M., Wylie, G. R., Higgins, B. A., Javitt, D. C., Schroeder, C. E., & Foxe, J. J. (2002). The spatiotemporal dynamics of illusory contour processing: combined high-density electrical mapping, source analysis, and functional magnetic resonance imaging. Journal of Neuroscience, 22, 5055–5073.
Oram, M. W., & Perrett, D. I. (1992). Time course of neural responses discriminating different views of the face and head. Journal of Neurophysiology, 68, 70–84.
Peterhans, E., & Von Der Heydt, R. (1989). Mechanisms of contour perception in monkey visual cortex II. Contour bridging gaps. Journal of Neuroscience, 9, 1749–1763.
Polat, U., Mizobe, K., Pettet, M. W., Kasamatsu, T., & Norcia, A. M. (1998). Collinear stimuli regulate visual responses depending on cell’s contrast threshold. Nature, 391, 580–584.
Polimeni, J., Balasubramanian, M., & Schwartz, E. L. (2006). Multi-area visuotopic map complexes in macaque striate and extra-striate cortex. Vision Research, 46, 3336–3359.
Polsky, A., Mel, B. W., & Schiller, J. (2004). Computational subunits in thin dendrites of pyramidal cells. Nature Neuroscience, 7, 621–627.
Raizada, R. D. S., & Grossberg, S. (2001). Context-sensitive bindings by the laminar circuits of V1 and V2: a unified model of perceptual grouping, attention and orientation contrast. Visual Cognition, 8, 431–466.
Raizada, R. D. S., & Grossberg, S. (2003). Towards a theory of the laminar architecture of cerebral cortex: Computational clues from the visual system. Cerebral Cortex, 13, 100–113.
Ringach, D. L., & Shapley, R. (1996). Spatial and temporal properties of illusory contours and amodal boundary completion. Vision Research, 36, 3037–3050.
Rosenberg, J. R., Amjad, A. M., Breeze, P., Brillinger, D. R., & Halliday, D. M. (1989). The Fourier approach to the identification of functional coupling between neuronal spike trains. Progress in Biophysics and Molecular Biology, 53, 1–31.
Salin, P., & Bullier, J. (1995). Corticocortical connections in the visual system: structure and function. Physiological Reviews, 75, 107–154.
Samonds, J. M., Zhou, Z., Bernard, M. R., & Bonds, A. B. (2006). Synchronous activity in visual cortex encodes collinear and cocircular contours. Journal of Neurophysiology, 95, 2602–2616.
Sandell, J. H., & Schiller, P. H. (1982). Effect of cooling area 18 on striate cortex cells in the squirrel monkey. Journal of Neurophysiology, 48, 38–48.
Sarpeshkar, R. (1998). Analog versus digital: extrapolating from electronics to neurobiology. Neural Computation, 10, 1601–1638.
Schmidt, K. E., Goebel, R., Löwel, S., & Singer, W. (1997). The perceptual grouping criterion of colinearity is reflected by anisotropies of connections in the primary visual cortex. European Journal of Neuroscience, 9, 1083–1089.
Segev, I. (1998). Cable and compartmental models of dendritic trees. In J. M. Bower & D. Beeman (Eds.), The book of genesis. New York: Springer-Verlag/TELOS.
Sekuler, A. B., & Palmer, S. E. (1992). Perception of partly occluded objects: a microgenetic analysis. Journal of Experimental Psychology: General, 121, 95–111.
Shmuel, A., Korman, M., Sterkin, A., Harel, M., Ullman, S., Malach, R., et al. (2005). Retinotopic axis specificity and selective clustering of feedback projections from V2 to V1 in the Owl monkey. Journal of Neuroscience, 23, 2117–2131.
Sillito, A. M., Jones, H. E., Gerstein, G. L., & West, D. C. (1994). Feature-linked synchronization of thalamic relay cell firing induced by feedback from the visual cortex. Nature, 369, 479–482.
Soriano, M., Spillmann, L., & Bach, M. (1996). The abutting grating illusion. Vision Research, 36, 109–116.
Spruston, N. (2008). Pyramidal neurons: dendritic structure and synaptic integration. Nature Reviews Neuroscience, 9, 206–221.
Stetter, D. D., Das, A., Bennett, J., & Gilbert, C. D. (2002). Lateral connectivity and contextual interactions in macaque primary visual cortex. Neuron, 36, 739–750.
Tallon, C., Bertrand, O., Bouchet, P., & Pernier, J. (1995). Gamma-range activity evoked by coherent visual stimuli in humans. European Journal of Neuroscience, 7, 1285–1291.
Tamas, G., Somogyi, P., & Buhl, E. H. (1998). Differentially interconnected networks of GABAergic interneurons in the visual cortex of the cat. Journal of Neuroscience, 18, 4255–4270.
Thomson, A. M., & Bannister, A. P. (2003). Interlaminar connections in the neocortex. Cerebral Cortex, 13, 5–14.
Thorpe, S., Delorme, A., & Van Rullen, R. (2001). Spike-based strategies for rapid processing. Neural Networks, 14, 715–725.
Traub, R. D., & Miles, R. (1991). Neuronal networks of the hippocampus. Cambridge: Cambridge University Press.
Tusa, R. J., Rosenquist, A. C., & Palmer, L. A. (1979). Retinotopic organization of areas 18 and 19 in the cat. Journal of Comparative Neurology, 185, 657–678.
VanRullen, R., Delorme, A., & Thorpe, S. J. (2001). Feed-forward contour integration in primary visual cortex based on asynchronous spike propagation. Neurocomputing, 38–40, 1–4.
VanRullen, R., Guyonneau, P., & Thorpe, S. J. (2005). Spike times make sense. Trends in Neuroscience, 28, 1–4.
Versace, M., Ames, H., Léveillé, J., Fortenberry, B., Mhatre, H., & Gorchetchnikov, A. (2008). KInNeSS: a modular framework for computational neuroscience. Neuroinformatics, 6, 291–309.
von der Heydt, R., Peterhans, E., & Baumgartner, G. (1984). Illusory contours and cortical neuron responses. Science, 224, 1260–1262.
von der Malsburg, C. (1981). The correlation theory of brain function. Göttingen: Internal Report 81-2, Dept. of Neurobiology, Max-Planck Institute for Biophysical Chemistry.
Yazdanbakhsh, A., & Grossberg, S. (2004). Fast synchronization of perceptual grouping in laminar visual cortical circuits. Neural Networks, 17, 707–718.
Yen, S.-C., & Finkel, L. H. (1998). Extraction of perceptually salient contours by striate cortical networks. Vision Research, 38, 719–741.
Yen, S.-C., Menschik, E. D., & Finkel, L. H. (1999). Perceptual grouping in striate cortical networks mediated by synchronization and desynchronization. Neurocomputing, 26–27, 609–616.
Yoshino, A., Kawamoto, M., Yoshida, T., Kobayashi, N., Shigemura, J., Takahashi, Y., et al. (2006). Activation time course of responses to illusory contours and salient region: a high-density electrical mapping comparison. Brain Research, 1071, 137–144.
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: B. A. Olshausen
Appendix
Appendix
1.1 Model
First the mathematical equations for single cell dynamics are described, followed by network equations.
1.2 Hodgkin-Huxley dynamics and compartmental equations
Neurons are implemented as either one or two compartments governed by cable equations (Segev 1998). Compartmental membrane potential V is governed by an equation of the form:
where I k refers to either synaptic, axial, injected or membrane channel currents, as explained below. Membrane capacitance C m is a function of compartment diameter (d) and length (l), and specific capacitance C M :
In accordance with general practice, we set \( {{\hbox{C}}_{\rm{M}}} = 1\mu {\hbox{F}}/{\hbox{c}}{{\hbox{m}}^2} \) (Koch 1999). Compartment dimensions d and l are the same for all neurons of a given layer. For clarity, dV / dt is noted dS / dt when the compartment is the soma, and by dD / dt when the compartment is a dendrite.
Somatic compartments are governed in part by Hodgkin-Huxley (1952) equations. To simplify notation, the collective influence of the leak, K+, and Na+ currents is denoted \( f\left( {S_i^j} \right) \), where \( S_i^j \) stands for the somatic membrane potential of unit i in layer j:
where g L , g K and g Na are the maximal conductances of the leak, K+, and Na+ channels respectively. E L , E K and E Na represent the reverse potentials of the three respective currents, with specific values shown in Tables 3 and 4. The short form in (3) is not used for dendritic compartments since, for simplicity, the latter only have a passive leak current term \( {g_L}\left( {D_i^j + \left| {{E_L}} \right|} \right) \). Gate variables n, m and h stand for the K + and Na + activating gates, and the Na + deactivating gate, respectively. The dynamical behavior of these gates is governed by the differential equation (where k = {n, m, k}):
The rate functions \( {\alpha_k}\left( {S_i^j} \right) \) and \( {\beta_k}\left( {S_i^j} \right) \) for the n, m and h gating variables are given in Eqs. (5)–(7), respectively. The parameters for these equations were adapted from Traub and Miles (1991) such that cells transition to spiking through a supercritical Andronov-Hopf bifurcation (Izhikevich 2007) and have a stable attractor for small external input:
and
Setting \( n(t) + h(t) \approx 0.84 \) and \( m = {m_\infty }(V) \), the (V,n)-phase plane (Izhikevich 2007) resulting from this choice of parameters is shown in Fig. 13. It can be seen that, with these parameters, model neurons behave as threshold units, firing only for sufficiently depolarizing input.
(a)–(c) Parameters used for Hodgkin-Huxley equations allow model neurons to lose stability through a supercritical Andronov-Hopf bifurcation with increasing current input strength (I). The presence of a stable attractor at low I ensures limited outward propagation of signals in the bipole network. (d) These settings also render model neurons analog sensitive in terms of firing rate (Hz)
For inter-compartmental currents, the actual axial conductance of neurons within layer j is denoted \( q_j^c \) and is defined by:
Note that the diameter \( d_j^c \) and length \( l_j^c \) are the dimensions of the compartment c towards which the current appears to be directed in the relevant equation. Thus, c is replaced by S in the case of the soma and by D in the case of dendrite. Parameter \( R_j^A \) denotes specific axial resistance for neurons in layer j.
Synaptic input \( I_{ij}^{kl} \) from unit i of layer k to unit j of layer l is modeled by:
where \( \delta_{ij}^{kl} \) stands for axonal delay for that particular synaptic connection. Function \( g_{ij}^{kl}\left( {t - \delta_{ij}^{kl},{t_n}} \right) \) is a double exponential (e.g. Köhn and Wörgötter 1998):
Constants τ r and τ f represent the rise-time and fall-time, respectively, of \( g_{ij}^{kl}\left( {t - \delta_{ij}^{kl},{t_n}} \right) \), which closely determines excitatory and inhibitory postsynaptic potentials (EPSP/IPSP) shape and duration (see Table 3). Constant p is set to ensure that \( g_{ij}^{kl}\left( \cdot \right) \in [0\;1] \). The summation and multiplication in (9) are taken over the set \( S_{ij}^{kl} \) of the last two spike times t n from unit i of layer k to unit j of layer l. Thus, keeping in mind that \( g_{ij}^{kl}\left( \cdot \right) \in [0\;1] \), the multiplicative term ensures that the aggregated conductances remain between 0 and 1, which implies that synaptic current \( I_{ij}^{kl} \) varies between 0 and \( w_{ij}^{kl} \). In the case of one-to-one connections, such that i = j, we abbreviate \( I_{ij}^{kl} \) by \( I_i^{kl} \) in Eq. (9).
The synaptic current resulting from the use of Eq. (9) is obtained by multiplying \( I_{ij}^{kl} \) with the voltage difference \( \left( {E - V_j^l} \right) \). Here, \( V_j^l \) is the membrane voltage of the post-synaptic compartment of cell j in layer l, and E is the driving potential specific to the type of synapse under consideration. For excitatory connections, E is set to a depolarizing value (0 mV). For inhibitory connections, E is set to a hyperpolarizing value (−60 or −70 mV).
1.3 Network equations
The neural network is composed of 1-dimensional arrays of layer 4 and layer 2/3 pyramidal cells and Left and Right interneurons. Each of the four layers contains 51 neurons. Figure 4 illustrates a representative diagram of the network where layer 2/3 cells and interneurons span 7 spatial locations. External input is provided at each layer 4 pyramidal cell. Each layer 4 cell projects to a single layer 2/3 cell. Horizontal excitatory connections originating from layer 2/3 cell span a total of 7 spatial locations. Left interneurons receive such horizontal connections only from layer 2/3 cells on their left. The reverse holds for Right interneurons. Left and Right interneurons at a given spatial location inhibit each other via recurrent connections. Both interneurons also inhibit the layer 2/3 cell at the same spatial location. These connections are described more precisely below.
All network equations are written with endogenous currents (ionic channels, inter-compartmental) on the left-hand side and exogenous currents (synaptic, injections) on the right-hand side.
1.4 Layer 4 pyramidal cells
Each layer 4 pyramidal cell is modeled as a single compartment (a soma) \( S_i^4 \) that receives externally injected input X i , where the latter is a scalar value for each neuron that is determined according to the simulation (see below):
1.5 Layer 2/3 pyramidal cells
Each layer 2/3 pyramidal cell is composed of one soma (\( S_i^2 \)) and one dendrite (\( D_i^2 \)) compartment. The dendritic compartment receives bottom-up excitatory input from one layer 4 pyramidal cell (\( I_i^{42} \)) and recurrent excitatory input from a Gaussian neighborhood of layer 2/3 pyramidal cells (\( \sum\limits_{m = i - 3}^{i + 3} {I_{mi}^{22}} \)):
The Gaussian distributed weight kernel and the axonal delay kernel implicit in \( I_{mi}^{22} \) are determined by Eqs. (13) and (14), respectively:
where m and i are the indices of the pre- and post- synaptic units respectively, and σ = 4.47. The maximal conductance, g max , is specific to the projection and values used for it are in Table 3.
The somatic potential is defined as follows: Input from layer 2/3 pyramidal cells is significantly delayed in time, reflecting the presence of slow conduction delays in layer 2/3 horizontal connections. The soma receives convergent inhibitory input from the Left and Right interneurons at the same spatial location (\( I_i^{L2} + I_i^{R2} \)):
Equation (15) implies that inhibitory interneurons have a decisive effect on the layer 2/3 pyramidal cell they innervate due to their direct action on the soma.
1.6 Layer 2/3 inhibitory interneurons
Layer 2/3 interneurons are divided into two groups according to whether they are to the Left or to the Right of the layer 2/3 pyramidal cell they innervate. Since the form of the differential equations is similar and all parameters are the same, only equations for Left interneurons are explicitly given here. Each Left layer 2/3 interneuron is composed of one soma (\( S_i^L \)) and one dendrite (\( D_i^L \)) compartment. The dendritic compartment receives excitatory input from a half-Gaussian neighborhood of Layer 2/3 pyramidal cells located to its left (\( \sum\limits_{m = i - 3}^{i - 1} {I_{mi}^{2L}} \)):
The synaptic weight and axonal delay parameters that define \( I_{mi}^{2L} \) are determined by Eqs. (13) and (14), respectively, with the additional constraint that it is set to 0 for m > i in the case of Left interneurons and for m < i in the case of Right interneurons.
Equation (17) governs the somatic membrane potential. The soma of a Left inhibitory interneuron only receives inhibitory input originating from the Right interneuron at the same spatial location:
1.7 Local field potential (LFP) calculations
Local field potentials are calculated in the KinNeSS software package according to the methodology described in Versace et al. (2008). Emplacement of the electrode is determined by choosing a random location within [10–200]μm of the middle spatial location of the array of pyramidal layer 2/3 cells and aligning the electrode shank with the orientation of the cells. The distance of that electrode to the remaining 50 cells in the layer was uniformly random within the [10–1,000]μm interval. The electrode is composed of five equally spaced electrode tips covering the entire length of layer 2/3 cells (i.e., sum of the dendrite and soma lengths). The LFP output did not differ significantly across electrode tips, so the respective outputs were averaged together in order to get a global estimate. The Fast Fourier Transform (FFT) was calculated on the averaged LFP thereby obtained.
1.8 Synchronization measure
The synchronization measure described in Rosenberg et al. (1989) was used to quantify the significance of the coupling in a selected pair of layer 2/3 pyramidal cells in Fig. 11 (right column). Each spike train is divided into L segments of length T. Let τ j represent spikes times, then the finite Fourier transform of the l th segment at frequency ω is given by:
The cross-spectrum between two spike trains (denoted a and b) is further given by:
where the bar indicates the complex conjugate. The squared magnitude of the estimated coherency between the two processes is defined as:
An upper 95% confidence limit to test for the presence of synchrony is given by \( 1 - {\left( {0.05} \right)^{1/\left( {L - 1} \right)}} \). This limit is plotted as a horizontal dashed line in Fig. 11. Values above the line indicate significant coupling in the frequency range indicated on the x-axis. Here L = 50 and simulations were run for 25,000 ms, such that T = 500 ms, yielding a frequency resolution of 2 Hz.
1.9 Parameters
1.9.1 Network
Biophysical parameters for synaptic connections and cells are given in Tables 3 and 4.
1.9.2 Weights and conduction delays
Horizontal weight kernels have a Gaussian shape as in Eq. (13) (half-Gaussian for connections reaching interneurons). The extent of the kernels is designed by dividing the maximum extent of horizontal connections by the width of a V1 hypercolumn (Yazdanbakhsh and Grossberg 2004). Assuming a 7 mm wide kernel and a 1 mm wide hypercolumn, the kernel size is set to 7 spatial locations.
Horizontal axonal conduction delay kernels are linearly dependent on distance, as in Eq. (14). The delay between neighboring hypercolumns is calculated by dividing the hypercolumn width by horizontal conduction speed. Recent estimates of horizontal conduction speed in both monkey V1 and cat area 17 put it at approximately 0.3 m/s (Bringuier et al. 1999; Girard et al. 2001; Hirsch and Gilbert 1991). Using a hypercolumn width of 1 mm, the conduction delay between neighboring hypercolumns is set to 3 ms; see Eq. (14).
1.9.3 Simulation protocol
All simulations were performed with KinNeSS (Versace et al. 2008). Unless mentioned otherwise, firing rates are measured once the network reaches a steady state, by counting the number of spikes in the last second of simulation. An integration time-step of 0.05 ms, 0.02 ms, or 0.01 ms was used to obtain numerically accurate results. All simulations took less than one hour on a dual 2 Ghz AMD Opteron workstation with 4 Gb of RAM running Linux. Unless mentioned otherwise, simulations were conducted for 2,000 ms of simulated time.
The results displayed in Figs. 6, 8 and 9 represent averages (and standard deviation for error bars) from nine different parameter settings where the maximal conductance of AMPA connections were varied along two dimensions. Specifically, maximal conductance of the layer 4–2/3 connection was varied within the set {0.048, 0.049, 0.05} mS/cm2 and maximal conductance of the layer 2/3-interneuron connection was varied within the set {0.036, 0.037, 0.038} mS/cm2. Results appeared qualitatively similar in all cases. The simulations in Fig. 7 correspond to the middle parameter configuration (i.e., values of 0.049 and 0.037 for respective parameters).
In simulations where this is relevant, stimulus contrast is defined as X i /0.0006, where X i is the current injected into layer 4 pyramidal cells. Thus, nonlinearities between stimulus contrast and current input to cortical cells are not included in the simulations, implying that the simulations of contrast-dependent data may arise totally due to properties of the bipole network.
1.9.4 Simulation of latency to first spike (Fig. 3(d))
The results displayed in Fig. 3(d) represents the difference between illusory and real contours in the latency of the first spike over the middle position of the contour, and for various current input strengths. Nonzero input was applied at locations 22 to 30 for real contours, whereas locations 25 to 27 were reset to zero for illusory contours. Current input was set to 0.0084, 0.015, 0.021, 0.03, 0.045, 0.06, 0.075, 0.09, 0.105 and 0.12 nA.
1.9.5 Simulation of bipole property (Fig. 5)
Nonzero input was applied to units at spatial locations 21, 22, 23, 29, 30 and 31 in the ID input array. Input strength at those locations was X i = 0.03 nA. The simulation was conducted for 2,000 ms and firing rates were calculated by monitoring spikes in the last 500 ms in order to ensure that the network has settled into a stable firing mode.
1.9.6 Short-range completion simulations (Fig. 6)
Each stimulus bar is represented as an input (X i ) to a single location in Layer 4. This is motivated by considerations of recent estimates of the cortical magnification factor (cmf) at 4° of eccentricity (Polimeni et al. 2006). Accordingly, a cmf of 2.7 mm/° gives approximately 1 mm of cortical extent to a 30′ stimulus bar as was used in the original study of Kapadia et al. (2000). However, 1–2 mm is the approximate diameter of one hypercolumn. Thus, a single stimulus bar is presented to a single location. This is also consistent with their adjustment of the length of the bars to the size of the CRFs. Stimulus contrasts of 20%, 30% and 50% were simulated by adjusting input strength to 0.012, 0.018 and 0.03 nA, respectively.
1.9.7 Simulations of long-range modulation (Fig. 7)
The size of the bar stimuli in Polat et al. (1998) match CRF size which is here mapped to 3 adjacent columns. The distance between flanking bars is set to 7 locations, which is the shortest distance for which no inward completion occurred for the set of parameters considered. This particular constraint is in accordance with the method used in the original paper of Polat et al. (1998), and serves the purpose of studying long-range modulation instead of short-range completion. The current inputs simulated were (in nA): 0.0036, 0.006, 0.0072, 0.0126, 0.021, 0.03, 0.06, 0.09 and 0.12.
1.9.8 Outward propagation simulations (Fig. 8)
In the original study of Crook et al. (2002), each stimulus bar was simulated as input to a single location. In the original study, the separation between the target cell recorded and the cell whose CRF receives the flanking stimulus was ∼2 mm. This corresponds to the approximate size of the cat’s hypercolumn width (Lund et al. 2003). Thus, in the simulations the target and flanking bars were presented at adjacent locations. The target input strength was set to 0.0096 nA and that of the flanker line was set to 0.06 nA in order to approach the 1-to-10 contrast ratio in the original study.
1.9.9 Horizontal summation simulations (Fig. 9)
Arrays of collinear Gabor patches are represented by low contrast stimuli in the simulations. This is meant to represent the fact that the patches used in this study are of smaller diameter than the measured CRF size, such that they do not produce maximal activation. The length of stimuli used is determined by using the cmf (.21 mm/°) reported by the authors multiplied by the length (in degrees) of the original stimuli (here 5, 10, 15, 18, 23, 28 and 33 degrees). The corresponding array lengths are: 1, 2, 3, 4, 5, 6 and 7 mm, where each mm corresponds to one location in our simulations, which is in the order of the size of a hypercolumn in the tree shrew (Bosking et al. 1997). For completeness, input stimuli are simulated at three contrast magnitudes by setting input strength to values of 0.0084, 0.0126 or 0.03 nA. The firing rate obtained is divided by the firing rate obtained for a 13-units long bar of high contrast (input strength set to 0.048 nA) simulated for the central parameter configuration in order to report quantities as relative activation with respect to a continuous bar of high contrast, consistent with the original study of Chisum et al. (2003).
1.9.10 Fast resynchronization simulations (Fig. 10)
The simulations of Fig. 10(a) and (b) were constructed by inserting a fixed random frame with input values ranging between 0 and 0.018 nA across spatial locations for the initial 100 ms and then switching to a homogeneous input stimulus for the remaining 900 ms. In the case of the full contour simulation (Fig. 10(a)), homogeneous input of magnitude 0.03 nA was applied to units 22 to 30. In the case of the illusory contour simulation (Fig. 10(b)), the same homogeneous input was applied to units 22, 23, 24, 28, 29 and 30. Membrane potential traces of layer 2/3 bipole cells were low-pass filtered with a Butterworth filter of order 4 to remove single spikes but preserve slow oscillations, which simplifies detection of phase synchrony. Visual inspection of traces revealed that bursts occurred during ups and silent periods during troughs of the resulting filtered signals. The oscillations displayed therefore reliably represent burst occurrences.
1.9.11 Oscillatory synchrony simulations (Fig. 11)
Simulations were run for 25,000 ms. The full bar consisted of a 9-units wide stimulus. Separate bars consisted of 3-units wide stimuli, separated by a 3-units wide gap. The pair of cells selected for recording had their CRF located in the middle of each bar and were thus separated by 5 hypercolumns. This reflects the arrangement in the experimental recordings by Gray et al. (1989) where the pair of cortical cells was separated by approximately 7 mm. Input strength was set to 0.03 nA. For the full bar and coherent bars condition, stimuli were presented with simultaneous injection in the layer 2/3 dendrites of 10 ms sub-threshold white noise frames of amplitude varying in the interval [0 0.003] nA. For the incoherent bar condition, each short bar was presented for a randomly determined period of 300–500 ms. Each bar presentation was followed by a noise-only period of 300–500 ms, whose purpose was to attenuate the periodicity artificially induced in the delta band by the slow alternation of stimulus bars. Note that, exclusion of these periods from the simulation did not change the synchronization profile of Fig. 11 (right) in the frequency bands of interest (mostly beta and gamma). Furthermore, the cross-correlograms reported in the middle column were shifted 100 ms in time to magnify the signal strength for the incoherent bar condition. Indeed, without this shift, the cross-correlogram output remains at 0 in the time frame considered for that condition, due to the inclusion of noise-only frames. However, the pattern of result remains the same when removing the shift. The coherence index \( {\left| R \right|^2} \) was calculated according to Eqs. (18)–(20).
1.9.12 LFP spectrum simulations (Fig. 12)
Simulations were run for 25,000 ms with 10 ms noise frames. Static input was set to 0.012 nA and time-varying noise magnitude spanned the interval [0 0.006] nA, such that the signal-to-noise ratio varied from 0 to 50%. The stimulus pattern simulated—i.e. a long bar, short bar, flankers-only bar, etc.—did not significantly affect the LFP power spectrum, whose peak varied in the upper 30 Hz to 50 Hz range. In the particular simulation shown in Fig. 10, the stimulus pattern consisted of two 8 units wide flankers separated by a 5 units wide gap, and noise magnitude was set to 0 (no noise condition).
1.9.13 Threshold and analog sensitive single-cells (Fig. 13)
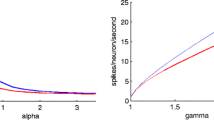
The plots A, B and C were generated from Eqs. (5)–(7) using the phase plane technique described in Izhikevich (2007), and for three representative current input levels (no current, low current, large depolarizing current). Plot D was generated by measuring the firing rate of a single neuron as the current input to that neuron was increased from 0 nA.
1.9.14 Funding
All authors were partially supported by CELEST, a National Science Foundation Science of Learning Center [SBE-0354378]; S.G. and M.V. were partially supported by the SyNAPSE program of the Defense Advanced Research Project Agency [HR001109-03-0001]; and S.G. and J.L. were also partially supported by the Defense Advanced Research Project Agency [HR001-09-C-0011].
Rights and permissions
About this article
Cite this article
Léveillé, J., Versace, M. & Grossberg, S. Running as fast as it can: How spiking dynamics form object groupings in the laminar circuits of visual cortex. J Comput Neurosci 28, 323–346 (2010). https://doi.org/10.1007/s10827-009-0211-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-009-0211-1