Abstract
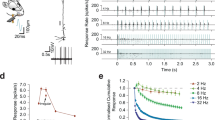
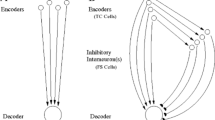
Recent works on the response of barrel neurons to periodic deflections of the rat vibrissae have shown that the stimulus velocity is encoded in the corti cal spike rate (Pinto et al., Journal of Neurophysiology, 83(3), 1158–1166, 2000; Arabzadeh et al., Journal of Neuroscience, 23(27), 9146–9154, 2003). Other studies have reported that repetitive pulse stimulation produces band-pass filtering of the barrel response rate centered around 7–10 Hz (Garabedian et al., Journal of Neurophysiology, 90, 1379–1391, 2003) whereas sinusoidal stimulation gives an increasing rate up to 350 Hz (Arabzadeh et al., Journal of Neuroscience, 23(27), 9146–9154, 2003). To explore the mechanisms underlying these results we propose a simple computational model consisting in an ensemble of cells in the ventro-posterior medial thalamic nucleus (VPm) encoding the stimulus velocity in the temporal profile of their response, connected to a single barrel cell through synapses showing short-term depression. With sinusoidal stimulation, encoding the velocity in VPm facilitates the response as the stimulus frequency increases and it causes the velocity to be encoded in the cortical rate in the frequency range 20–100 Hz. Synaptic depression does not suppress the response with sinusoidal stimulation but it produces a band-pass behavior using repetitive pulses. We also found that the passive properties of the cell membrane eventually suppress the response to sinusoidal stimulation at high frequencies, something not observed experimentally. We argue that network effects not included here must be important in sustaining the response at those frequencies.









Similar content being viewed by others
Notes
The difference between this synaptic model and averaged synaptic response models of STD (Abbott et al. 1997; Tsodyks and Markram 1997), is that release and recovery are stochastic in one case and deterministic in the other. Neglecting the stochastic nature of the transmission leads to an underestimation of the fluctuations of the synaptic current and the post-synaptic response rate (de la Rocha and Parga 2005).
References
Abbott, L. F., Varela, J. A., Sen, K., & Nelson, S. B. (1997). Synaptic depression and cortical gain control. Science, 275, 179–80.
Ahissar, E., Sosnik, R., & Haidarliu, S. (2000). Transformation from temporal to rate coding in somatosensory thalamocortical system. Nature, 406, 302–306.
Andermann, M. L., Ritt, J., Neimark, M. A., & Moore, C. I. (2004). Neural correlates of vibrissa resonance; band-pass and somatotopic representation of high-frequency stimuli. Neuron, 42(3), 451–63.
Arabzadeh, E., Petersen, R. S., & Diamond, M. E. (2003). Encoding of whisker vibration by rat barrel cortex neurons: Implications for texture discrimination. Journal of Neuroscience, 23(27), 9146–9154.
Bazhenov, M., Rulkov, N. F., Fellous, J.-M., & Timofeev, I. (2005). Role of network dynamics in shaping spike timing reliability. Physical Review E, 72, 041903.
Beierlein, M., Gibson, J. R., & Connors, B. W. (2003). Two dynamically distinct inhibitory networks in layer 4 of the neocortex. Journal of Neurophysiology, 90(5), 2987–3000.
Borg-Graham, L., Monier, C., & Fregnac, Y. (1998). Visual input evokes transient and strong shunting inhibition in visual cortical neurons. Nature, 393, 369–373.
Brunel, N., Chance, F. S., Fourcaud, N., & Abbott, L. F. (2001). Effects of synaptic noise and filtering on the frequency response of spiking neurons. Physical Review Letters, 86(10), 2186–2189.
Bruno, R. M., & Sakmann, B. (2006). Cortex is driven by weak but synchronously active thalamocortical synapses. Nature, 312, 1622–1627.
Carandini, M., Heeger, D. J., & Senn, W. (2001). A synaptic explanation of suppression in visual cortex. Journal of Neuroscience, 22, 10053–10065.
Carvell, G., & Simons, D. (1990). Biometric analyses of vibrissal tactile discrimination in the rat. Journal of Neuroscience, 10(8), 2638–2648.
Castro-Alamancos, M. A. (1997). Short term plasticity in the thalamocortical pathways: Cellular mechanisms and functional roles. Reviews in the Neurosciences, 20, 95–116.
Castro-Alamancos, M. A. (2002). Different temporal processing of sensory inputs in the rat thalamus during quiescent and information processing states in vivo. Journal of Physiology (Lond), 539(2), 567–578.
Castro-Alamancos, M. A., & Oldford, E. (2002). Cortical sensory suppression during arousal is due to the activity-dependent depression of thalamocortical synapses. Journal of Physiology (Lond), 541, 319–331.
Chance, F. S., Abbott, L., & Reyes, A. D. (2002). Gain modulation from background synaptic input. Neuron, 35, 773–782.
Chance, F. S., Nelson, S. B., & Abbott, L. F. (1998). Synaptic depression and the temporal response characteristics of v1 cells. Journal of Neuroscience, 12, 4785–4799.
Chung, S., Li, X., & Nelson, S. B. (2002). Short-term depression at thalamocortical synapses contributes to rapid adaptation of cortical sensory responses in vivo. Neuron, 34, 437–446.
de la Rocha, J., Nevado, A., & Parga, N. (2002). Information transmission by stochastic synapses with short-term depression: Neural coding and optimization. Neucomputing, 44–46, 85–90.
de la Rocha, J., & Parga, N. (2005). Short-term synaptic depression causes a non-monotonic response to correlated stimuli. Journal of Neuroscience, 25(37), 8416–8431.
Deschenes, M., Timofeeva, E., & Lavallee, P. (2003). The relay of high-frequency sensory signals in the whisker-to-barreloid pathway. Journal of Neuroscience, 23(17), 6778–6787.
Eggermont, J. J. (1999). The magnitude and phase of temporal modulation transfer functions in cat auditory cortex. Journal of Neuroscience, 19(7), 2780–2788.
Fanselow, E. E., & Nicolelis, M. A. L. (1999). Behavioral modulation of tactile responses in the rat somatosensory system. Journal of Neuroscience, 19(17), 7603–7616.
Fleidervish, I. A., Binshtok, A. M., & Gutnick, M. J. (1998). Functionally distinct NMDA receptors mediate horizontal connectivity within layer 4 of mouse barrel cortex. Neuron, 21(5), 1055–1065.
Garabedian, C. E., Jones, S. R., Merzenich, M. M., Dale, A., & Moore, C. I. (2003). Band-pass response properties of rat si neurons. Journal of Neurophysiology, 90, 1379–1391.
Gerstner, W. (2000). Population dynamics of spiking neurons: Fast transients, asynchronous states, and locking. Neural Computation, 12(1), 43–89.
Gerstner, W., & Kistler, W. (2002). Spiking neuron models: Single neuron, populations, plasticity. Cambridge University Press.
Gil, Z., Connors, B. W., & Amitai, Y. (1999). Efficacy of thalamocortical and intracortical synaptic connections: Quanta, innervation, and reliability. Neuron, 23, 385–397.
Goldberg, J., & Brown, P. (1969). Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: Some physiological mechanisms of sound localization. Journal of Neurophysiology, 32, 613–636.
Golomb, D., Ahissar, E., & Kleinfeld, D. (2006). Coding of stimulus frequency by latency in thalamic networks through the interplay of GABAB-mediated feedback and stimulus shape. Journal of Neurophysiology, 95(3), 1735–1750.
Hartings, J. A., & Simons, D. J. (1998). Thalamic relay of afferent responses to 1- to 12-Hz whisker stimulation in the rat. Journal of Neurophysiology, 80(2), 1016–1019.
Hartings, J. A., Temereanca, S., & Simons, D. J. (2003). Processing of periodic whisker deflections by neurons in the ventroposterior medial and thalamic reticular nuclei. Journal of Neurophysiology, 90(5), 3087–3094.
Hirsch, J. A., Alonso, J.-M., Reid, R. C., & Martinez, L. M. (1998). Synaptic integration in striate cortical simple cells. Journal of Neuroscience, 18, 9517–9528.
Hutcheon, B., & Yarom, Y. (2000). Resonance, oscillations and the intrinsic frequency preferences of neurons. Trends in Neuroscience, 23(5), 216–222.
Khatri, V., Hartings, J. A., & Simons, D. J. (2004). Adaptation in thalamic barreloid and cortical barrel neurons to periodic whisker deflections varying in frequency and velocity. Journal of Neurophysiology, 92(6), 3244–3254.
Koch, C. (1999). Biophysics of computation: Information processing in single neurons. Oxford University Press.
Land, P. W., Buffer, S. A., & Yaskosky, J. D. (1995). Barreloids in adult rat thalamus: Three-dimensional architecture and relationship to somatosensory cortical barrels. The Journal of Comparative Neurology, 355(4), 573–588.
Lichtenstein, S., Carvell, G., & Simons, D. (1990). Responses of rat trigeminal ganglion neurons to movements of vibrissae in different directions. Somatosensory & Motor Research, 7, 47–65.
Lin, K. K., Shea-Brown, E., & Young, L.-S. (2007). Reliability of coupled oscillators I: Two-oscillator systems. Submitted to Journal of Nonlinear Science. Avaliable online: http://arxiv.org/abs/0708.3061.
Lu, T., Liang, L., & Wang, X. (2001). Temporal and rate representations of time-varying signals in the auditory cortex of awake primates. Nature Neuroscience, 4, 1131–1138.
Magleby, K. L. (1987). Short-term changes in synaptic efficacy. In G. Edelman, W. Gall, & W. Cowan (Eds.), Synaptic function (pp. 21–56). New York: Wiley.
Matveev, V., & Wang, X.-J. (2000). Implications of all-or-none synaptic transmission and short-term depression beyond vesicle depletion: A computational study. Journal of Neuroscience, 20, 1575–1588.
Moore, C. I. (2004). Frequency-dependent processing in the vibrissa sensory system. Journal of Neurophysiology, 91, 2390–2399.
Neimark, M. A., Andermann, M. L., Hopfield, J. J., & Moore, C. I. (2003). Vibrissa resonance as a transduction mechanism for tactile encoding. Journal of Neuroscience, 23(16), 6499–6509.
Pinto, D. J., Brumberg, J. C., & Simons, D. J. (2000). Circuit dynamics and coding strategies in rodent somatosensory cortex. Journal of Neurophysiology, 83(3), 1158–1166.
Pinto, D. J., Hartings, J. A., Brumberg, J. C., & Simons, D. J. (2003). Cortical damping: Analysis of thalamocortical response transformations in rodent barrel cortex. Cerebral Cortex, 13, 33–44.
Ricciardi, L. M. (1977). Diffusion processes and related topics in biology. Berlin: Springer.
Rieke, F., Warland, D., de Ruyter van Steveninck, R. R., & Bialek, W. (1996). Spikes: Exploring the neural code. Cambridge, MA: MIT Press.
Shoykhet, M., Doherty, D., & Simons, D. (2000). Coding of deflection velocity and amplitude by whisker primary afferent neurons: Implications for higher level processing. Somatosensory & Motor Research, 17, 171–18.
Simons, D. J. (1978). Response properties of vibrissa units in rat si somatosensory neocortex. Journal of Neurophysiology, 41, 798–820.
Sosnik, R., Haidarliu, S., & Ahissar, E. (2001). Temporal frequency of whisker movement. I. Representations in brain stem and thalamus. Journal of Neurophysiology, 86(1), 339–353.
Stratford, K., Tarczy-Hornoch, K., Martin, K., Bannister, N., & Jack, J. (1996). Excitatory synaptic inputs to spiny stellate cells in cat visual cortex. Nature, 382, 258–261.
Svirskis, G., & Rinzel, J. (2003). Influence of subthreshold nonlinearities on signal-to-noise ratio and timing precision for small signals in neurons: Minimal model analysis. Network: Computation in Neural Systems, 14(1), 137–150.
Swadlow, H. (1989). Efferent neurons and suspected interneurons in s1 vibrissa cortex of the awake rabbit: Evidence from cross-correlation, microstimulation, and latencies to peripheral sensory stimulation. Journal of Physiology, 62, 288–308.
Swadlow, H. A., & Gusev, A. G. (2001). The impact of ‘bursting’ thalamic impulses at a neocortical synapse. Nature Neuroscience, 4(4), 401–408.
Tsodyks, M. V., & Markram, H. (1997). The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability. Proceedings of the National Academy of Sciences of the United States of America, 94, 719–723.
Varga, C., Sik, A., Lavallee, P., & Deschenes, M. (2002). Dendroarchitecture of relay cells in thalamic barreloids: A substrate for cross-whisker modulation. Journal of Neuroscience, 22(14), 6186–6194.
Vere-Jones, D. (1966). Simple stochastic models for the release of quanta of transmitter from a nerve terminal. Australian Journal of Statistics, 8, 53–63.
Wang, X.-J. (1999). Fast burst firing and short-term synaptic plasticity: A model of neocortical chattering neurons. Neuroscience, 89(2), 347–362.
Welker, W. (1964). Analysis of sniffing of the albino rat. Behavior, 12, 223–244.
Welker,W. I., Johnson Jr., J. I., & Pubols Jr., B. H. (1964). Some morphological and physiological characteristics of the somatic sensory system in raccoons. American Zoologist, 136, 75–94.
Zucker, R. S., & Regehr, W. G. (2002). Short-term synaptic plasticity. Annual Review of Physiology, 64, 355–405.
Acknowledgements
The research presented in this manuscript was supported by a grant from the Spanish Ministry of Education and Science (FIS 2006-09294). JR was also funded by a Postdoctoral Fellowship from the same institution. We thank Miguel Maraval and Alfonso Renart for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: Xiao-Jing Wang
Appendices
Appendix 1
1.1 Values of the parameters
We take the synaptic parameters from in vitro and in vivo studies. In agreement with in vitro data obtained by Gil et al. (1999) we take as mean quantal EPSP size is J/C m ≃ 0.35 mV with a coefficient of variation Δ ≃ 0.25; mean number of release sites M = 7; release probability U = 0.8. In the absence of any spontaneous activity in the thalamus these values produce a mean EPSP amplitude < PSP > = M U J/C m = 1.96 mV. Experiments in anesthetized and sedated rats have recently reported smaller mean amplitudes which depend on the animal state: for anesthetized animals mean = 1.94 mV (median 1.3 mV) whereas for sedated animals the mean = 0.49 mV (median 0.24 mV; Bruno and Sakmann 2006). These variation was accompanied with a difference in the spontaneous thalamic rate (ν 0 = 1.0 Hz for anesthetized and 5.4 Hz for sedated (Bruno and Sakmann 2006)) suggesting that TC synapses are continuously depressed in vivo (Castro-Alamancos 2002; Bruno and Sakmann 2006). This continuous depression is expected to be larger in awake animals were spontaneous thalamic firing is even higher (~10 Hz; Fanselow and Nicolelis 1999; Swadlow and Gusev 2001). Finally, a different study in anesthetized rats has shown that the depressed TC EPSP amplitude recovers to rest values exponentially with a time constant τ v ~5 s (Chung et al. 2002). Had we used this value, which is almost two orders of magnitude larger than that measured in in vitro recordings of TC synapses in the visual system (Stratford et al. 1996), and spontaneous thalamic rates like those found in vivo (~5–10 Hz), we would have obtained an average TC EPSP lower than 0.05 mV. We therefore set τ v = 300 ms to reproduce average TC EPSP like those found in (Bruno and Sakmann 2006) when the spontaneous thalamic rate was 5 Hz.
We consider N = 85 thalamic neurons impinging onto the single target barrel cell based on an estimate of 200 cells per barreloid (Land et al. 1995; Varga et al. 2002) and a TC convergence ratio of 0.43 (Bruno and Sakmann 2006). Other parameters of the cortical cell model are: τ m = 1 − 10 ms, θ = 10–17 mV, H = 6–10 mV, τ ref = 2 ms, C m = 100 pF and E L = 0. Background parameters: M E = 3, M I = 6, J E /C m = 0.2 mV, J I /C m = − 0.4 mV, U E = U I = 0.4, \(\nu_E=5\;\textrm{ms}^{-1}\), \(\nu_I=1\;\textrm{ms}^{-1}\). The excitatory background rate being five times larger than the inhibitory background rate, reflects the approximate ratio of one inhibitory cell every five excitatory cells found in the cortex.
Appendix 2
1.1 The temporal contrast
The temporal contrast is defined as (Pinto et al. 2000):
It measures in spiking rate units the mean firing rate of the early part of the response. Parameterizing the thalamic CPSTH with a Gamma function given by:
the temporal contrast can be evaluated easily: The numerator equals 0.4 e C Σ, while the denominator is obtained by solving the equation:
which performing the integral becomes
which yields to t = 1.37 Σ, giving finally:
that is, it is simply proportional to the Gamma amplitude. Now, modeling the temporal contrast dependence on the velocity obtained by Pinto et al. (2000) using this Gamma parameterization implies that as the stimulus velocity increases the VPm response has to vary (1) increasing its amplitude C and (2) decreasing Σ so that the product CΣ remains fixed. Doing so, the temporal contrast depends on the velocity while the spike count is independent of it.
Rights and permissions
About this article
Cite this article
de la Rocha, J., Parga, N. Thalamocortical transformations of periodic stimuli: the effect of stimulus velocity and synaptic short-term depression in the vibrissa–barrel system. J Comput Neurosci 25, 122–140 (2008). https://doi.org/10.1007/s10827-007-0068-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-007-0068-0