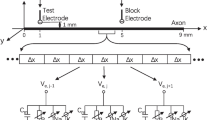
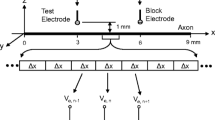
Abstract
The influences of stimulation frequency and temperature on mechanisms of nerve conduction block induced by high-frequency biphasic electrical current were investigated using a lumped circuit model of the myelinated axon based on Schwarz and Eikhof (SE) equations. The simulation analysis showed that a temperature–frequency relationship was determined by the axonal membrane dynamics (i.e. how fast the ion channels can open or close.). At a certain temperature, the axonal conduction block always occurred when the period of biphasic stimulation was smaller than the action potential duration (APD). When the temperature decreased from 37 to 15°C, the membrane dynamics slowed down resulting in an APD increase from 0.4 to 2.4 ms accompanied by a decrease in the minimal blocking frequency from 4 to 0.5 kHz. The simulation results also indicated that as the stimulation frequency increased the mechanism of conduction block changed from a cathodal/anodal block to a block dependent upon continuous activation of potassium channels. Understanding the interaction between the minimal blocking frequency and temperature could promote a better understanding of the mechanisms of high frequency induced axonal conduction block and the clinical application of this method for blocking nerve conduction.











Similar content being viewed by others
References
Agnew, W. F., & McCreery, D. H. (1990). Neural prostheses: Fundamental studies. Englewood Cliffs, NJ: Prentice Hall.
Bazhenov, M., Timofeev, I., Steriade, M., & Sejnowski, T. J. (2004). Potassium model for slow (2–3 Hz) in vivo neocotical paroxysmal oscillations. Journal of Neurophysiology, 92, 1116–1132.
Bhadra, N., Bhadra, N., Kilgore, K. L., & Gustafson, K. J. (2006). High frequency electrical conduction block of pudendal nerve. Journal of Neural Engineering, 3, 180–187.
Bhadra, N., & Kilgore, K. L. (2005). High-frequency electrical conduction block of mammalian peripheral motor nerve. Muscle Nerve, 32, 782–790.
Bikson, M., Lian, J., Hahn, P. J., Stacey, W. C., Sciortino, C., & Durand, D. M. (2001). Suppression of epileptiform activity by high frequency sinusoidal fields in rat hippocampal slices. Journal of Physiology (London), 531, 181–191.
Bowman, B. R., & McNeal, D. R. (1986). Response of single alpha motoneurons to high frequency pulse train: Firing behavior and conduction block phenomenon. Applied Neurophysiology, 49, 121–138.
Boyce, W. E., & Diprima, R. C. (1997). Elementary differential equations and boundary value problems (6th ed.). New York: Wiley.
Chiu, S. Y., Ritchie, J. M., Rogart, R. B., & Stagg D. (1979). A quantitative description of membrane currents in rabbit myelinated nerve. Journal of Physiology (London), 292, 149–166.
Elwassif, M. M., Kong, Q., Vazquez, M., & Bikson M. (2006). Bio-heat transfer model of deep brain stimulation-induced temperature changes. Journal of Neural Engineering, 3, 306–315.
Frankenhaeuser, B., & Huxley, A. F. (1964). The action potential in the myelinated nerve fibre of Xenopus Laevis as computed on the basis of voltage clamp data. Journal of Physiology (London), 171, 302–315.
Hodgkin, A. L., & Huxley, A. F. (1952). A quantitative description of membrane current and its application to conduction and excitation in nerve. Journal of Physiology (London), 117, 500–544.
Kager, H., Wadman, W. J., & Somjen, G. G. (2000). Simulated seizures and spreading depression in a neuron model incorporating interstitial space and ion concentrations. Journal of Neurophysiology, 84, 495–512.
Kager, H., Wadman, W. J., & Somjen, G. G. (2002). Condition for the triggering of spreading depression studied with computer simulations. Journal of Neurophysiology, 88, 2700–2712.
Kilgore, K. L., & Bhadra N. (2004). Nerve conduction block utilising high-frequency alternating current. Medical & Biological Engineering & Computing, 42, 394–406.
McIntyre, C. C., Richardson, A. G., & Grill, W. M. (2002). Modeling the excitability of mammalian nerve fibers: Influence of afterpotentials on the recovery cycle. Journal of Neurophysiology, 87, 995–1006.
Nashold, B. S., Goldner, J. L., Mullen, J. B., & Bright, D. S. (1982). Long-term pain control by direct peripheral-nerve stimulation. Journal of Bone and Joint Surgery, 64A, 1–10.
Rattay, F., & Aberham M. (1993). Modeling axon membranes for functional electrical stimulation. IEEE Transactions on Biomedical Engineering, 40, 1201–1209.
Reboul, J., & Rosenblueth A. (1939). The action of alternating currents upon the electrical excitability of nerve. American Journal of Physiology, 125, 205–215.
Renganathan, M., Cummins, T. R., & Waxman, S. G. (2001). Contribution of Nav 1.8 sodium channels to action potential electrogenesis in DRG neurons. Journal of Neurophysiology, 86, 629–640.
Roper, J., & Schwarz, J. R. (1989). Heterogeneous distribution of fast and slow potassium channels in myelinated rat nerve fibres. Journal of Physiology (London), 416, 93–110.
Rosenblueth, A., & Reboul J. (1939). The blocking and deblocking effects of alternating currents on nerve. American Journal of Physiology, 125, 251–264.
Schwarz, J. R., & Eikhof G. (1987). Na currents and action potentials in rat myelinated nerve fibres at 20 and 37°C. Pflugers Archiv, 409, 569–577.
Schwarz, J. R., Glassmeier, G., Cooper, E. C., Kao, T. C., Nodera, H., Tabuena, D., et al. (2006). KCNQ channels mediate Iks, a slow K+ current regulating excitability in the rat node of Ranvier. Journal of Physiology, 573, 17–34.
Schwarz, J. R., Reid, G., & Bostock H. (1995). Action potentials and membrane currents in the human node of Ranvier. Pflugers Archiv, 430, 283–292.
Tai, C., de Groat, W. C., & Roppolo, J. R. (2005a). Simulation analysis of conduction block in unmyelinated axons induced by high-frequency biphasic electrical currents. IEEE Transactions on Biomedical Engineering, 52, 1323–1332.
Tai, C., de Groat, W. C., & Roppolo, J. R. (2005b). Simulation of nerve block by high-frequency sinusoidal electrical current based on the Hodgkin–Huxley model. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 13, 415–422.
Tai, C., Roppolo, J. R., & de Groat, W. C. (2004). Block of external urethral sphincter contraction by high frequency electrical stimulation of pudendal nerve. Journal of Urology, 172, 2069–2072.
Tai, C., Roppolo, J. R., & de Groat, W. C. (2005c). Response of external urethral sphincter to high frequency biphasic electrical stimulation of pudendal nerve. Journal of Urology, 174, 782–786.
Tanner, J. A. (1962). Reversible blocking of nerve conduction by alternating-current excitation. Nature, 195, 712–713.
Williamson, R. P., & Andrews, B. J. (2005). Localized electrical nerve blocking. IEEE Transactions on Biomedical Engineering, 52, 362–370.
Zhang, X., Roppolo, J. R., de Groat, W. C., & Tai C. (2006a). Simulation analysis of conduction block in myelinated axons induced by high-frequency biphasic rectangular pulses. IEEE Transactions on Biomedical Engineering, 53, 1433–1436.
Zhang, X., Roppolo, J. R., de Groat, W. C., Tai C (2006b) Mechanism of nerve conduction block induced by high-frequency biphasic electrical currents. IEEE Transactions on Biomedical Engineering, 53, 2445–2454.
Acknowledgement
This work is supported by the NIH under grants 1R01-DK-068566-01 and 1R01-NS-051671-01A1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: John R. Huguenard
Appendix
Appendix
The ionic current I i,,n at nth node is described as:
where P Na (0.00328 cm/s) and P K (0.000134 cm/s) are the ionic permeabilities for sodium and potassium currents, respectively; g L (86 kΩ−1 cm−2) is the maximum conductance for leakage current. VL (0 mV) is the reduced equilibrium membrane potential for leakage ions, in which the resting membrane potential V rest (−78 mV) has been subtracted. [Na] i (8.71 mmol/l) and [Na] o (154 mmol/l) are sodium concentrations inside and outside the axon membrane. [K] i (155 mmol/l) and [K] o (5.9 mmol/l) are potassium concentrations inside and outside the axon membrane. F (96,485 C/mole) is Faraday constant. R (8,314.4 mJ K−1 mol−1) is gas constant. m, h and n are dimensionless variables, whose values always change between 0 and 1. m and h represent activation and inactivation of sodium channels, whereas n represents activation of potassium channels.
The evolution equations for m, h and n are the following:
and
where T is the temperature used in the simulation study (in °K). The initial values for m, h and n (when V n = 0 mV) are 0.0077, 0.76 and 0.0267, respectively.
Rights and permissions
About this article
Cite this article
Wang, J., Shen, B., Roppolo, J.R. et al. Influence of frequency and temperature on the mechanisms of nerve conduction block induced by high-frequency biphasic electrical current. J Comput Neurosci 24, 195–206 (2008). https://doi.org/10.1007/s10827-007-0050-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-007-0050-x