Abstract
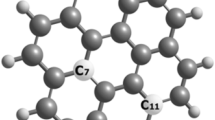
Density functional theory as well as molecular mechanics force field (MMFF94) techniques are used to study intermolecular/intramolecular interactions, band offsets, physicochemical correlations, and nonlinear optical properties for 12 designed homo- and hetero-pyrrole-graphene quantum dots (PGQDs). Also, force field energy, minimum and maximum projection area, Van der Waals volume and 3D surface area, and total polar surface area for homo- and hetero-PGQDs are calculated. In addition, UV–Vis absorption spectra for homo- and hetero-PGQDs are computed. The interfacial electrons carry through functionalized GQDs with pyrroles bearings will help in screening the desired applications for proposed PGQDs as optoelectronic and memory switches devices.






Similar content being viewed by others

Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical or time limitations.
References
Qiu, Y., Zhang, Y.: A Ademiloye and ZJCMS Wu: molecular dynamics simulations of single-layer and rotated double-layer graphene sheets under a high velocity impact by fullerene. Comput. Mater. Sci. 182, 109 (2020)
Cui, T., Mukherjee, S., Sudeep, P.M., Colas, G., Najafi, F., Tam, J., Ajayan, P.M., Singh, C.V., Sun, Y., Filleter, T.: Fatigue of graphene. Nat. Mater. 19(4), 405 (2020)
Ye, X., Zhu, Y., Jiang, H., Yue, Z., Wang, L., Wan, Z., Jia, C.: Constructing molecules supported holey graphene sheets framework in compact graphene film to achieve synergistic effect for ion transport and high gravimetric/volumetric capacitances. J. Power Sources 441, 227167 (2019)
Han, X.Y., Zhao, D.L., Meng, W.J., Yang, H.X., Zhao, M., Duan, Y.J., Tian, X.M.: Graphene caging silicon nanoparticles anchored on graphene sheets for high performance Li-ion batteries. Appl. Sur. Sci. 484, 11 (2019)
You, R., Liu, Y.Q., Hao, Y.L., Han, D.D., Zhang, Y.L., You, Z.: Laser fabrication of graphene-based flexible electronics. Adv. Mater. 32(15), 1901981 (2020)
Yan, C., Lee, P.S.: Stretchable energy storage and conversion devices. Small 10(17), 3443 (2014)
Wang, B., Ruan, T., Chen, Y., Jin, F., Peng, L., Zhou, Y., Wang, D., Dou, S.: Graphene-based composites for electrochemical energy storage. Energy Storage Mater. 24, 22 (2020)
Sadeghzadeh, S.: Nanoparticle mass detection by single and multilayer graphene sheets: theory and simulations. Appl. Math. Model. 40(17–18), 7862 (2016)
Shen, C., Chu, Y., Wu, Q., Li, N., Wang, S., Zhao, Y., Tang, J., Liu, J., Tian, J., Watanabe, K.: Correlated states in twisted double bilayer graphene. Nat. Phys. 16(5), 520 (2020)
Xue, C., Gao, H., Hu, Y., Hu, G.: Hyperelastic characteristics of graphene natural rubber composites and reinforcement and toughening mechanisms at multi-scale. Compos. Struct. 228, 111365 (2019)
Yang, H., Yuan, L., Yao, X., Fang, D.: Piezoresistive response of graphene rubber composites considering the tunneling effect. J. Mech. Phys. Solids 139, 103943 (2020)
Böhm, S.: Graphene against corrosion. Nature Nanotechnol. 9(10), 741 (2014)
Hao, S., Wang, J., Lavorgna, M., Fei, G., Wang, Z., Xia, H.: Constructing 3D graphene network in rubber nanocomposite via liquid-phase redispersion and self-assembly. ACS Appl. Mater. Interfaces. 12(8), 9682 (2020)
Mao, H.Y., Laurent, S., Chen, W., Akhavan, O., Imani, M., Ashkarran, A.A., Mahmoudi, M.: Graphene: promises, facts, opportunities, and challenges in nanomedicine. Chem. Rev. 113(5), 3407 (2013)
Alinejad, A., Raissi, H., Hashemzadeh, H.: Understanding co-loading of doxorubicin and camptothecin on graphene and folic acid-conjugated graphene for targeting drug delivery: classical MD simulation and DFT calculation. J. Biomol. Struct. Dyn. 38(9), 2737 (2020)
Figueroa, T., Aguayo, C., Fernández, K.: Design and characterization of chitosan-graphene oxide nanocomposites for the delivery of proanthocyanidins. Int. J. Nanomed. 15, 1229 (2020)
Raghavan, A., Sarkar, S., Nagappagari, L.R., Bojja, S., MuthukondaVenkatakrishnan, S., Ghosh, S.: Decoration of graphene quantum dots on TiO2 nanostructures: photosensitizer and cocatalyst role for enhanced hydrogen generation. Ind. Eng. Chem. Res. 59(29), 13060 (2020)
Sharma, V., Kagdada, H.L., Wang, J., Jha, P.K.: Hydrogen adsorption on pristine and platinum decorated graphene quantum dot: A first principle study. Int. J. Hydrogen Energy 45(44), 23977 (2020)
Hu, Y., Chen, W., Lei, T., Jiao, Y., Wang, H., Wang, X., Xiong, J.: Graphene quantum dots as the nucleation sites and interfacial regulator to suppress lithium dendrites for high-loading lithium-sulfur battery. Nano Energy 68, 104373 (2020)
Kang, S., Jeong, Y.K., Ryu, J.H., Son, Y., Kim, W.R., Lee, B., Kim, K.M.: Pulsed laser ablation based synthetic route for nitrogen-doped graphene quantum dots using graphite flakes. Appl. Surf. Sci. 506, 144998 (2020)
Liu, Q., Sun, J., Gao, K., Chen, N., Sun, X., Ti, D., Qu, L.: Graphene quantum dots for energy storage and conversion: from fabrication to applications. Mater. Chem. Front. 4(2), 421 (2020)
Liu, H., Li, C., Qian, Y., Hu, L., Fang, J., Tong, W., Wang, H.: Magnetic-induced graphene quantum dots for imaging-guided photothermal therapy in the second near-infrared window. Biomaterials 232, 119700 (2020)
Sarkar, S., Ramanathan, N., Gopi, R., Sundararajan, K.: Pyrrole multimers and pyrrole-acetylene hydrogen bonded complexes studied in N2 and para-H2 matrixes using matrix isolation infrared spectroscopy and ab initio computations. J. Mol. Struct. 1149, 387 (2017)
Ramanathan, N., Sankaran, K., Sundararajan, K.: Nitrogen: a new class of π-bonding partner in hetero π-stacking interaction. J. Phys. Chem. A 121(47), 9081 (2017)
Sarkar, S., Ramanathan, N., Sundararajan, K.: Effect of methyl substitution on the N–H··· O interaction in complexes of pyrrole with water, methanol, and dimethyl ether: matrix isolation infrared spectroscopy and ab initio computational studies. J. Phys. Chem. A 122(9), 2445 (2018)
Sarkar, S., Ramanathan, N., Sruthi, P.K., Sundararajan, K.: Computational and experimental evidence of N-H… π and cooperative πN… π∗ interactions in pyrrole… benzene and pyrrole… ethylene heterodimers at low temperatures. J. Mol. Struct. 1209, 127 (2020)
Müller, S., Paulus, J., Mattay, J., Ihmels, H., Dodero, V.I., Sewald, N.: Photocontrolled DNA minor groove interactions of imidazole/pyrrole polyamides. Beilstein J. Organic Chem. 16(1), 60 (2020)
Sarkar, S., Ramanathan, N., Sruthi, P.K., Sundararajan, K.: Conformations of diethyl ether and its interaction with pyrrole at low temperatures. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 213, 361 (2019)
Petrov, A.A., Fakhurtdinova, A.R., Khachatrian, A.A., Rakipov, I.T., Akhmadiyarov, A.A., Solomonov, B.N.: The intermolecular interactions of methanol, pyrrole and chloroform in a binary solvent. Thermochim. Acta 689, 178640 (2020)
Kalluvettukuzhy, N.K., Pagidi, S., Prasad Nandi, R., Thilagar, P.: Exploiting N− H–π interactions in 2-(dimesitylboraneyl)-1H-pyrrole for luminescence enhancement. Asian J. Organic Chem. 9(4), 644 (2020)
Le, D.T.H., Tri, N.N., Man, N.T.H., Trung, N.T.: A theoretical study of X-H∙∙∙ π and X∙∙∙ π interactions in the complexes of furan, thiophene, pyrrole and hydrogen halides. Vietnam J. Chem. 58(2), 151 (2020)
Vandana, M., Ashokkumar, S., Vijeth, H., Yesappa, L., Devendrappa, H.: Synthesis and characterization of polypyrrole-graphene quantum dots nanocomposites for supercapacitor application. In: AIP Conference Proceedings, (2115), p. 030535. AIP Publishing LLC, City (2019)
Sert, Y., Sreenivasa, S., Doğan, H., Manojkumar, K.E., Suchetan, P.A., Ucun, F.: FT-IR, Laser-Raman spectra and quantum chemical calculations of methyl 4-(trifluoromethyl)-1H-pyrrole-3-carboxylate-A DFT approach. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc 127, 122 (2014)
El-Mansy, M.A.M.: Quantum chemical studies on structural, vibrational, nonlinear optical properties and chemical reactivity of indigo carmine dye. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 183, 284 (2017)
El-Nahass, M.M., Kamel, M.A., El-Barbary, A.A., El-Mansy, M.A.M., Ibrahim, M.: FT-IR spectroscopic analyses of 3-methyl-5-pyrazolone (MP). Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 111, 37 (2013)
Shoormasti, A.S., Abbasi, A., Orouji, A.A.: Using energy band engineering to improve heterojunction solar cells efficiency. Optik 218, 165243 (2020)
El-Barbary, A.A., El-Nahass, M.M., Kamel, M.A., El-Mansy, M.A.M.: FT-IR, FT-Raman spectra and ab initio HF, DFT vibrational analysis of P-methyl acetanilide. J. Appl. Sci. Res. 11, 1977 (2009)
Ibrahim, M., El-Barbary, A.A., El-Nahass, M.M., Kamel, M.A., El-Mansy, M.A.M., Asiri, A.M.: On the spectroscopic analyses of (E)-3-(dicyclopropyl methylene)-dihydro-4-[1-(2, 5 dimethylfuran-3-yl) ethylidene] furan-2, 5-dione. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 87, 202 (2012)
El-Mansy, M.A.M., El-Nahass, M.M., Khusayfan, N.M., El-Menyawy, E.M.: DFT approach for FT-IR spectra and HOMO–LUMO energy gap for N-(p-dimethylaminobenzylidene)-p-nitroaniline (DBN). Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 111, 217 (2013)
Ibrahim, M., El-Nahass, M., Kamel, M., El-Barbary, A., Wagner, B., El-Mansy, M.: On the spectroscopic analyses of thioindigo dye. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 113, 332 (2013)
Soliman, H.S., Eid, K.M., Ali, H.A.M., El-Mansy, M.A.M., Atef, S.M.: FT-IR spectroscopic analyses of 2-(2-furanylmethylene) propanedinitrile. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 105, 545 (2013)
Valsaraj, K.T., Thibodeaux, L.J.: Relationships between micelle-water and octanol-water partition constants for hydrophobic organics of environmental interest. Water Res. 23(2), 183 (1989)
Amézqueta, S., Subirats, X., Fuguet, E., Rosés, M., Ràfols, C.: Octanol-Water Partition CONSTANT, in Liquid-Phase Extraction, p. 183. Elsevier (2020)
Padron, J.A., Carrasco, R., Pellon, R.F.: Molecular descriptor based on a molar refractivity partition using Randic-type graph-theoretical invariant. J. Pharm. Pharmaceut. Sci 5(3), 258 (2002)
Borges, N.M., Kenny, P.W., Montanari, C.A., Prokopczyk, I.M., Ribeiro, J.F.R., Rocha, J.R., Sartori, G.R.: The influence of hydrogen bonding on partition coefficients. J. Comput. Aided Mol. Des. 31(2), 163 (2017)
Viswanadhan, V.N., Ghose, A.K., Revankar, G.R., Robins, R.K.: Atomic physicochemical parameters for three dimensional structure directed quantitative structure-activity relationships. 4. Additional parameters for hydrophobic and dispersive interactions and their application for an automated superposition of certain naturally occurring nucleoside antibiotics. J. Chem. Inf. Comput. Sci. 29, 163 (1989)
Du, Q., Mezey, P.G., Chou, K.C.: Heuristic molecular lipophilicity potential (HMLP): A 2D-QSAR study to LADH of molecular family pyrazole and derivatives. J. Comput. Chem.. 26(5), 461 (2005)
Jahan, R., Bodratti, A.M., Tsianou, M., Alexandridis, P.: Biosurfactants, natural alternatives to synthetic surfactants: Physicochemical properties and applications. Adv. Colloid Interface Sci. 275, 102 (2020)
Liu, Q., Guan, J., Qin, L., Zhang, X., Mao, S.: Physicochemical properties affecting the fate of nanoparticles in pulmonary drug delivery. Drug Discov. Today 25(1), 150–159 (2020)
Szafrański, P.W., Trybula, M.E., Kasza, P., Cegła, M.T.: Following the oxidation state of organosulfur compounds with NMR: experimental data versus DFT calculations and database-powered NMR prediction. J. Mol. Struct. 1202, 127 (2020)
Ali, M.R., Mezei, M.: Observation of quantum signature in rivastigmine chemical bond break-up and quantum energetics, spectral studies of anti-Alzheimer inhibitors. J. Biomol. Struct. Dyn. 39(1), 118–128 (2021)
Ali, I., Mahmood, L.M., Mehdar, Y.T., Aboul-Enein, H.Y., Said, M.A.: Synthesis, characterization, simulation, DNA binding and anticancer activities of Co (II), Cu (II), Ni (II) and Zn (II) complexes of a Schiff base containing o-hydroxyl group nitrogen ligand. Inorg. Chem. Commun. 118, 108004 (2020)
Dimova, V., Jankulovska, M.S.: Application of topological descriptors in QSAR modeling: substituted hydrazones used as a model system. Lett. Drug Design Disc. 17(3), 253 (2020)
Ertl, P., Rohde, B., Selzer, P.: Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 43(20), 3714 (2000)
Shityakov, S., Neuhaus, W., Dandekar, T., Förster, C.: Analyzing molecular polar surface descriptors to predict blood-brain barrier permeation. J. Neuroimmune Pharmacol. 7, 55 (2012)
Frisch, G.W.T.M.J., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery, J.A., Jr., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., Pople, J.A.: Gaussian09. Revision A Inc., Wallingford CT (2009)
Frisch, A., Dennington, R., Keith, T., Millam, J., Nielsen, A., Holder, A., Hiscocks, J.: Gauss view version 5 user manual. Gaussian Inc., Wallingford, CT, USA. (2009)
Halgren, T.A.: Merck molecular force field I basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 17(5–6), 490 (1996)
Kaminski, G., Jorgensen, W.L.: Performance of the AMBER94, MMFF94, and OPLS-AA force fields for modeling organic liquids. J. Phys. Chem. 100(46), 18010 (1996)
Csizmadia, P.: MarvinSketch and MarvinView: molecule applets for the World Wide Web (1999).
Olah, M.: Molecular fragment volume calculation for QSAR studies by the MTD-method. Rev. Roum. Chim. 45(12), 1123–1125 (2000)
Bode, J.W.: Reactor ChemAxon Ltd., Maramaros koz 2/a, Budapest, 1037 Hungary. www.chemaxon.com. Contact ChemAxon for pricing information ACS Publications, City (2004)
Liao, C., Sitzmann, M., Pugliese, A., Nicklaus, M.C.: Software and resources for computational medicinal chemistry. Fut. Med. Chem. 3(8), 1057 (2011)
Prasad, S., Aljaafreh, M.J., Masilamani, V., AlSalhi, M.S., Mujamammi, W.M.: Time-resolved excited state dynamics of super-exciplex in the coumarin dye laser. J. Mol. Liq. 315, 113 (2020)
Schmiermund, T.: DIE AVOGADRO-KONSTANTE: entstehung einer naturkonstante. Springer, New York (2020)
Schmiermund, T.: Die Avogadro-Konstante
Wu, C., Liu, S., Zhang, S., Yang, Z.: Molcontroller: a VMD graphical user interface featuring molecule manipulation. J. Chem. Inf. Model. 60(10), 5126 (2020)
Wu, C., Liu, S., Zhang, S., & Yang, Z.: Molcontroller: a VMD graphical user interface for manipulating molecules (2020)
Author information
Authors and Affiliations
Contributions
MAMEl-M contributed to conceptualization, methodology, analysis, writing-original draft, editing, and reviewing; AS contributed to methodology, analysis, writing, and editing; WOY contributed to calculations, data curing, analysis, writing, and editing; KFK contributed to calculations, data curing, analysis, writing, editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Table 6.
Rights and permissions
About this article
Cite this article
El-Mansy, M.A.M., Suvitha, A., Osman, W. et al. Exploring the electronic and optical absorption properties for homo- and hetero-pyrrole-graphene quantum dots. J Comput Electron 20, 2387–2402 (2021). https://doi.org/10.1007/s10825-021-01773-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10825-021-01773-w