Abstract
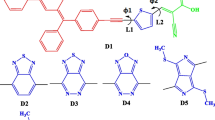
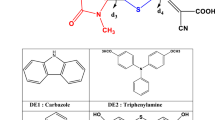
Since, the organic dyes that harness sunlight are generally considered as the heart of the dye sensitized solar cells (DSSC), the present study was carried out with the aim to design heterocyclic azo dyes that can be potentially used in DSSC application. Hereby, the analysis based on density functional theory (DFT) and time-dependent DFT calculations of the geometries, electronic structures and absorption spectra of the dyes before and after binding to titanium oxide \((\hbox {TiO}_{2})\) were carried out and investigated in detail. The data obtained from these analyses were then used to determine the open-circuit photovoltage \((\hbox {V}_\mathrm{OC})\), and to measure the important parameters such as the light harvesting efficiency (LHE) and the electron injection efficiency associated with the short-circuit photocurrent density \((\hbox {J}_\mathrm{SC})\). Our investigation reveals that all dyes showed absorbance in the visible region (469–521 nm) with high oscillator strength \((f)\) (1.076–1.564) and LHE (0.9176–0.973). Moreover, we found that the dyes after binding to titanium oxide displayed slightly red-shifted absorption (475–527 nm) with improved oscillator strength \((f)\) (1.121–1.664) and LHE (0.921–0.979). In addition, all dyes showed high \(\hbox {V}_\mathrm{OC}\) (1.068–2.232 eV) and high driving force for the electron injection, thus leading to the larger \(\hbox {J}_\mathrm{SC}\). Our findings indicate that the heterocyclic azo dyes investigated in the current study can display better light to power conversion efficiency if used in the DSSC system, where the origin or their better performance can be attributed to the high \(\hbox {J}_\mathrm{SC}\) and \(\hbox {V}_\mathrm{OC}\) values found for these potential dyes. Based on the detailed study and investigation, we believe that the theoretical criteria used in the present study can be employed as an initial screening tool not merely to assess the properties of other organic dyes, but also to potentially design the organic azo dyes for their potential application in the DSSC systems.




Similar content being viewed by others
References
O’Regan, B., Gratzel, M.: A low-cost, high-efficiency solar cell based on dye-sensitized colloidal \(\text{ TiO }_{2}\) films. Nature 353, 737–740 (1991)
Abdullah, M.I., Janjua, M.R.S.A., Nazar, M.F., Mahmood, A.: Quantum chemical designing of efficient TC4-based sensitizers by modification of auxiliary donor and \(\uppi \)-spacer. Bull. Chem. Soc. Jpn. 86, 1272–1281 (2013)
Boschloo, G., Hagfeldt, A.: Characteristics of the iodide/triiodide redox mediator in dye-sensitized solar cells. Acc. Chem. Res. 42, 1819–1826 (2009)
Nazeeruddin, M.K., Angelis, F.D., Fantacci, S., Selloni, A., Viscardi, G., Liska, P., Ito, S., Takeru, B., Gratzel, M.: Combined experimental and DFT–TDDFT computational study of photoelectrochemical cell ruthenium sensitizers. J. Am. Chem. Soc. 127, 16835–16847 (2005)
Abdullah, M.I., Janjua, M.R.S.A., Mahmood, A., Ali, S., Ali, M.: Quantum chemical designing of efficient sensitizers for dye sensitized solar cells. Bull. Korean Chem. Soc. 34, 2093–2098 (2013)
Mishra, A., Fischer, M.K.R., Bäuerle, P.: Metal-free organic dyes for dye-sensitized solar cells: from structure: property relationships to design rules. Angew. Chem. Int. Ed. Engl. 48, 2474–2499 (2009)
Zhang, G.L., Bala, H., Cheng, Y.M., Shi, D., Lv, X.J., Yu, Q.J., Wang, P.: High efficiency and stable dye-sensitized solar cells with an organic chromophore featuring a binary \(\uppi \)-conjugated spacer. Chem. Commun. 16, 2198–2200 (2009)
Choi, H., Choi, H., Paek, S., Song, K., Kang, M.-S., Ko, J.: Novel organic sensitizers with a quinoline unit for efficient dye-sensitized solar cells. Bull. Korean Chem. Soc. 31, 125–132 (2010)
Li, Y.-T., Chen, C.-L., Hsu, Y.-Y., Hsu, H.-C., Chi, Y., Chen, B.-S., Liu, W.-H., Lai, C.-H., Lin, T.-Y., Chou, P.-T.: Donor–acceptor organic sensitizers assembled with isoxazole or its derivative 3-oxopropanenitrile. Tetrahedron 66, 4223–4229 (2010)
Irfan, A., Al-Sehemi, A.: Quantum chemical study in the direction to design efficient donor-bridge-acceptor triphenylamine sensitizers with improved electron injection. J. Mol. Model. 18, 4893–4900 (2012)
Al-Eid, M., Limb, S.H., Park, K.-W., Fitzpatrick, B., Han, C.-H., Kwak, K., Hong, K.: Graeme Cooke Facile synthesis of metal-free organic dyes featuring a thienylethynyl spacer for dye sensitized solar cells. Dyes Pigments 104, 197–203 (2014)
Lee, W., Choi, J., Namgoong, J.W., Kim, S.H., Sun, K.C., Jeong, S.H., Yoo, K., Ko, M.J., Kim, J.P.: The effect of five-membered heterocyclic bridges and ethoxyphenyl substitution on the performance of phenoxazine-based dye-sensitized solar cells. Dyes Pigments 104, 185–193 (2014)
Preat, J., Jacquemin, D., Perpete, E.A.: Towards new efficient dye-sensitised solar cells. Energy Environ. Sci. 3, 891–904 (2010)
Tai, C.-K., Chen, Y.-J., Chang, H.-W., Yeh, P.-L., Wang, B.-C.: DFT and TD-DFT investigations of metal-free dye sensitizers for solar cells: effects of electron donors and \(\uppi \)-conjugated linker. Comput. Theor. Chem. 971, 42–50 (2011)
Abbotto, A., Barolo, C., Bellotto, L., De Angelis, F., Gratzel, M., Manfredi, N., Marinzi, C., Fantacci, S., Yum, J., Nazeeruddin, M.K.: Heteroaromatic conjugated bipyridine based ruthenium sensitizer for efficient dye-sensitized solar cells. Chem. Commun. 42:5318–5320 (2008)
Towns, A.D.: Developments in azo disperse dyes derived from heterocyclic diazo components. Dyes Pigments 42, 3–25 (1999)
Yesodha, S.K., Pillai, C.K.S., Tsutsumi, N.: Stable polymeric materials for nonlinear optics: a review based on azobenzene systems. Prog. Polym. Sci. 29, 45–74 (2004)
Matharu, A., Jeeva, S., Huddleston, P.R., Ramanujam, P.S.: Synthesis and optical storage properties of a thiophene-based holographic recording medium. J. Mater. Chem. 17, 4477–4482 (2007)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery Jr., J.A., Peralta, P.E., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, K.N., Staroverov, V.N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, N.J., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, O., Ortiz, J.V., Cioslowski, J., Fox, D.J.: Gaussian 09, Revision A.1. Gaussian Inc., Wallingford (2009)
Dreuw, A., Head-Gordon, M.: Single-reference ab initio methods for the calculation of excited states of large molecules. Chem. Rev. 105, 4009–4037 (2005)
Yanai, T., Tew, D.P., Handy, N.C.: A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 393, 51–57 (2004)
Zhang, J., Li, H.-B., Wu, Y., Geng, Y., Duan, Y.-A., Liao, Y., Su, Z.-M.: Chem. J. Chin. Univ. 32, 1343–1348 (2011)
Barone, V., Cossi, M.: Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 102, 1995–2001 (1998)
Qin, P., Yang, X.C., Chen, R.K., Sun, L.C., Marinado, T., Edvinsson, T., Boschloo, G., Hagfeldt, G.: Influence of \(\uppi \)-conjugation units in organic dyes for dye-sensitized solar cells. J. Phys. Chem. C 111, 1853–1860 (2007)
Gratzel, M.: Photoelectrochemical cells. Nature 414, 338–344 (2001)
Zhang, G.L., Bai, Y., Li, R.Z., Shi, D., Wenger, S., Zakeeruddin, S.M., Gratzel, M., Wang, P.: Employ a bisthienothiophene linker to construct an organic chromophorefor efficient and stable dye-sensitized solar cells. Energy Environ. Sci. 2, 92–95 (2009)
Qin, C., Clark, A.E.: DFT characterization of the optical and redox properties of natural pigments relevant to dye-sensitized solar cells. Chem. Phys. Lett. 438, 26–30 (2007)
Hagfeldt, A., Graetzel, M.: Light-induced redox reactions in nanocrystalline systems. Chem. Rev. 95, 49–68 (1995)
Persson, P., Bergström, R., Ojamäe, L., Lunell, S.: Quantum-chemical studies of metal oxides for photoelectrochemical applications. Adv. Quant. Chem. 41, 203–263 (2002)
Pearson, R.G.: Absolute electronegativity and hardness: application to inorganic chemistry. Inorg. Chem. 27, 734–740 (1988)
Zhang, C.R., Liu, Z.J., Chen, Y.H., Chen, H.S., Wu, Y.Z., Yuan, L.H.: DFT and TDDFT study on organic dye sensitizers D5, DST and DSS for solar cells. J. Mol. Struct. 899, 86–93 (2009)
Jung, H.S., Lee, J.K.: Dye sensitized solar cells for economically viable photovoltaicsystems. J. Phys. Chem. Lett. 4, 1682 (2013)
Choi, H., Kamat, P.V.: Know thy nano neighbor. Plasmonic versus electron charging effects of metal nanoparticles in dye-sensitized solar cells. ACS Nano 6, 4418 (2012)
Acknowledgments
The authors would like to sincerely appreciate the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project no RGP-VPP-255.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahmood, A., Khan, S.UD. & Rana, U.A. Theoretical designing of novel heterocyclic azo dyes for dye sensitized solar cells. J Comput Electron 13, 1033–1041 (2014). https://doi.org/10.1007/s10825-014-0628-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10825-014-0628-2