Abstract
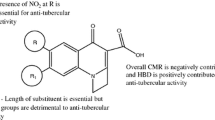
QSAR analysis of a set of 96 heterocyclics with antifungal activity was performed. The results reveals that a pyridine ring is more favorable than benzene as the 6-membered ring, for high activity, but thiazole is unfavorable as the 5-membered ring relative to imidazole or oxazole. Methylene is the spacer leading to the highest activity. The descriptors used are indicator variables, which account for identity of substituent, lipophilicity and volume of substituent, and total polarizability. Unlike previously reported results for this data set, our fits do not exceed the limitations set by the nature of the data itself.


Similar content being viewed by others
Notes
aClare, B.W. (2008) Martha.zip, available free of charge from the site: http://mirrors.uwa.edu.au/mirrors/weboffice/martha/
Hyperchem,6.0:Hypercube Inc,1115 NW 4th Street, Gainesville, Florida 32601–4256 USA
References
St-Georgiev V (2000) Curr Drug Targets 1:261. doi:10.2174/1389450003349209
Meyers FH, Jawetz E, Goldfien A (1976) Review of medical pharmacology, 5th edn. Lange Medical Pub, Los Altos, Calif
Yalcin I, Oren I, Temiz O, Sener EA (2000) Acta Biochim Pol 47:481
Rees JR, Pinner RW, Hajjeh RA (1998) Clin Infect Dis 27:1138. doi:10.1086/514975
Polak A (1999) Mycoses 42:355. doi:10.1046/j.1439-0507.1999.00475.x
Fostel JM, Lartey PA (2000) Drug Discov Today 5:25. doi:10.1016/S1359-6446(99)01430-0
Tafi A, Costi R, Botta M, Di Santo R, Corelli F, Massa S et al (2002) J Med Chem 45:2720. doi:10.1021/jm011087h
Chan JH, Hong JS, Kuyper LF, Baccanari DP, Joyner SS, Tansik RL et al (1995) J Med Chem 38:3608. doi:10.1021/jm00018a021
Elnima EI, Zubair MU, Al-Badr AA (1981) Antimicrob Agents Chemother 19:29
Goker H, Kus C, Boykin DW, Yildizc S, Altanlar N (2002) Bioorg Med Chem 10:2589–2596. doi:10.1016/S0968-0896(02)00103-7
Yildiz-Oren I, Yalcin I, Aki-Sener E, Ucarturk N (2004) Eur J Med Chem 39:291. doi:10.1016/j.ejmech.2003.11.014
Garci′a-Domenech, R., Ri′os-Santamarina, I., Catala′, A., Calabuig, C., del Castillo, L.,Ga′lvez, J. (2003) THEOCHEM 624:97
Hasegawa K, Deushi T, Yaegashi O, Miyashita Y, Sasaki S (1995) Eur J Med Chem 30:569. doi:10.1016/0223-5234(96)88271-7
Mghazli S, Jaouad A, Mansour M, Villemin D, Cherqaoui D (2001) Chemosphere 43: 385–390
Duchowicz PR, Vitale MG, Castro EA, Fernandez M, Caballero J (2007) Bioorg Med Chem 15:2680–2689. doi:10.1016/j.bmc.2007.01.039
Clare BW (2002) J Comput Aided Mol Des 16:611. doi:10.1023/A:1021966231380
Clare BW, Supuran CT (2005) Bioorg Med Chem 13:2197. doi:10.1016/j.bmc.2004.12.055
Deeb O, Alfalah S, Clare BW (2006) J Enzyme Inhib Med Chem 22:277. doi:10.1080/14756360601161966
Deeb O, Clare BW (2007) Chem Biol Drug Des 70:437. doi:10.1111/j.1747-0285.2007.00578.x
Stewart JJP (1990) QCPE Bull 10:86
MOPAC 93,Fujitsu Ltd.,Tokyo, Japan
Clare BW, Supuran CT (2005) J Chem Inf Model 45:1385. doi:10.1021/ci050191v
Kishida K, Manabe R (1980) Med J Osaka Univ 30:95
Ursu O, Costescu A, Diudea MV (2006) Croat Chem Acta 79:483
Topliss JG, Costello RJ (1972) J Med Chem 15:1066. doi:10.1021/jm00280a017
Topliss JG, Edwards RJ (1979) J Med Chem 22:1238. doi:10.1021/jm00196a017
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
The following supplementary material is available for this article:
Rights and permissions
About this article
Cite this article
Deeb, O., Clare, B.W. QSAR of heterocyclic antifungal agents by flip regression. J Comput Aided Mol Des 22, 885–895 (2008). https://doi.org/10.1007/s10822-008-9223-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-008-9223-6