Abstract
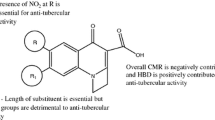
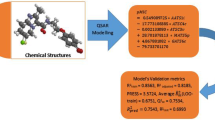
The CORAL software based on the optimal descriptors calculated with simplified molecular input-line entry system was used to build up quantitatively structure-activity relationship for the minimal inhibition concentrations against (i) Mycobacterium tuberculosis H37RV ATCC 27294 and (ii) M. tuberculosis drug resistant clinical isolate. The predictive potential of these models has been checked up with three random splits into the training, calibration, and validation sets. Although all models are quantitative, it has been found that splitting have apparent influence on the statistical quality of these models. Thus, the thesis “QSAR is a random event” is confirmed in this work.
Similar content being viewed by others
References
ACD/ChemSketch (2016) Advanced Chemistry Development, Toronto, Canada. http://www.acdlabs.com/products/draw_nom/draw/chemsketch/. Accessed 20 June 2016
Achary PGR (2014a) Simplified molecular input line entry system-based optimal descriptors: QSAR modelling for voltage-gated potassium channel subunit Kv7.2. SAR QSAR Environ Res 25:73–90
Achary PGR (2014b) QSPR modelling of dielectric constants of π-conjugated organic compounds by means of the CORAL software. SAR QSAR Environ Res 25:507–526
Calligaro GL, Moodley L, Symons G, Dheda K (2014) The medical and surgical treatment of drug-resistant tuberculosis. J Thorac Dis 6:186–195
Cassano A, Robinson RLM, Palczewska A, Puzyn T, Gajewicz A, Tran L, Manganelli S, Cronin MTD (2016) Comparing the CORAL and random forest approaches for modelling the in vitro cytotoxicity of silica nanomaterials. ATLA Altern Lab Anim 44:533–556
Connolly MY (2010) Quantitative drug design, a critical introduction, 2nd edn. CRC Press, Boca Raton
CORAL (2016) http://www.insilico.eu/CORAL. Accessed 20 June 2016
Demmer ChS, Bunch L (2015) Benzoxazoles and oxazolopyridines in medicinal chemistry studies. Eur J Med Chem 97:778–785
Ertan-Bolelli T, Yildiz I, Ozgen-Ozgacar S (2016) Synthesis, molecular docking and antimicrobial evaluation of novel benzoxazole derivatives. Med Chem Res 25:553–567
Fatemi MH, Malekzadeh H (2015) CORAL: Predictions of retention indices of volatiles in cooking rice using representation of the molecular structure obtained by combination of SMILES and graph approaches. J Iran Chem Soc 12:405–412
Garro Martinez JC, Duchowicz PR, Estrada MR, Zamarbide GN, Castro EA (2011) QSAR study and molecular design of open-chain enaminones as anticonvulsant agents. Int J Mol Sci 12:9354–9368
Ghaedi A (2015) Predicting the cytotoxicity of ionic liquids using QSAR model based on SMILES optimal descriptors. J Mol Liq 208:269–279
Heidari A, Fatemi MH (2017) A theoretical approach to model and predict the adsorption coefficients of some small aromatic molecules on carbon nanotube. J Chinese Chem Soc 64:289–295
Ibezim E, Duchowicz PR, Ortiz EV, Castro EA (2012) QSAR on aryl-piperazine derivatives with activity on malaria. Chemom Intell Lab Syst 110:81–88
Islam MA, Pillay TS (2016) Simplified molecular input line entry system-based descriptors in QSAR modeling for HIV-protease inhibitors. Chemometr Intell Lab Syst 153:67–74
Kumar A, Chauhan S (2017a) QSAR differential model for prediction of SIRT1 modulation using monte carlo method. Drug Res 67:156–162
Kumar A, Chauhan S (2017b) Use of the monte carlo method for OECD principles-guided QSAR modeling of SIRT1 inhibitors. Arch Pharm 350, article no. e1600268
Li Q, Ding X, Si H, Gao H (2014) QSAR model based on SMILES of inhibitory rate of 2, 3-diarylpropenoic acids on AKR1C3. Chemometr Intell Lab Syst 139:132–138
Mullen LMA, Duchowicz PR, Castro EA (2011) QSAR treatment on a new class of triphenylmethyl-containing compounds as potent anticancer agents. Chemom Intell Lab Syst 107:269–275
Poce G, Cocozza M, Consalvi S, Biava M (2014) SAR analysis of new anti-TB drugs currently in pre-clinical and clinical development. Eur J Med Chem 2014; 86:335–351
Rana DN, Chhabria MT, Shah NK, Brahmkshatriya PS (2014) Pharmacophore combination as a useful strategy to discover new antitubercular agents. Med Chem Res 23:370–381
Siddiqi N, Siddiqi MI (2014) Recent advances in QSAR-based identification and design of anti-tubercular agents. Curr Pharm Des 20:4418–4426
Sokolović D, Aleksić D, Milenković V, Karaleić S, Mitić D, Kocić J, Mekić B, Veselinović JB, Veselinović AM (2016a) QSAR modeling of bis-quinolinium and bis-isoquinolinium compounds as acetylcholine esterase inhibitors based on the Monte Carlo method—the implication for Myasthenia gravis treatment. Med Chem Res 25:2989–2998
Sokolović D, Ranković J, Stanković V, Stefanović R, Karaleić S, Mekić B, Milenković V, Kocić J, Veselinović AM (2017) QSAR study of dipeptidyl peptidase-4 inhibitors based on the Monte Carlo method. Med Chem Res 26:796–804
Sokolović D, Stanković V, Toskić D, Lilić L, Ranković G, Ranković J, Nedin-Ranković G, Veselinović AM (2016b) Monte Carlo-based QSAR modeling of dimeric pyridinium compounds and drug design of new potent acetylcholine esterase inhibitors for potential therapy of myasthenia gravis. Struct Chem 27:1511–1519
Toropov AA, Toropova AP, Benfenati E, Fanelli R (2016) QSAR as a random event: Selecting of the molecular structure for potential anti-tuberculosis agents. Anti-Infect Agents 14:3–10
Toropov AA, Toropova AP, Benfenati E, Gini G, Fanelli R (2013a) The definition of the molecular structure for potential anti-malaria agents by the Monte Carlo method. Struct Chem 24:1369–1381
Toropov AA, Toropova AP, Benfenati E, Gini G, Leszczynska D, Leszczynski J (2010) QSAR analysis of 1,4-dihydro-4-oxo-1-(2-thiazolyl)-1,8-naphthyridines exhibiting anticancer activity by optimal SMILES-based descriptors. J Math Chem 47:647–666
Toropov AA, Toropova AP, Benfenati E, Nicolotti O, Carotti A, Nesmerak K, Veselinović AM, Veselinović JB, Duchowicz PR, Bacelo D, Castro EA, Rasulev BF, Leszczynska D, Leszczynski J (2015) QSPR/QSAR analyses by means of the CORAL software: results, challenges, perspectives. In: Roy K (ed) Quantitative structure-activity relationships in drug design, predictive toxicology and risk assessment. IGI Global, Hershey, pp 560–585
Toropov AA, Toropova AP, Puzyn T, Benfenati E, Gini G, Leszczynska D, Leszczynksi J (2013b) QSAR as a random event: Models for nanoparticles uptake in PaCa2 cancer cells. Chemosphere 92:31–37
Toropova AP, Toropov AA, Benfenati E, Gini G (2011c) Co-evolutions of correlations for QSAR of toxicity of organometallic and inorganic substances. An unexpected good prediction based on a model that seems untrustworthy. Chemom Intell Lab Syst 105:215–219
Toropova AP, Toropov AA, Benfenati E, Gini G, Leszczynska D, Leszczynski J (2011a) CORAL: Quantitative structure - activity relationship models for estimating toxicity of organic compounds in rats. J Comput Chem 32:2727–2733
Toropova AP, Toropov AA, Diaza RG, Benfenati E, Gini G (2011b) Analysis of the co-evolutions of correlations as a tool for QSAR-modeling of carcinogenicity. An unexpected good prediction based on a model that seems untrustworthy. Cent Eur J Chem 9:165–174
Toropova AP, Toropov AA, Martyanov SE, Benfenati E, Gini G, Leszczynska D, Leszczynski J (2012) CORAL: QSAR modeling of toxicity of organic chemicals towards Daphnia magna. Chemom Intell Lab Syst 110:177–181
Veselinović AM, Veselinović JB, Živković JV, Nikolić GM (2015) Application of smiles notation based optimal descriptors in drug discovery and design. Cur Top Med Chem 15:1768–1779
Worachartcheewan A, Nantasenamat C, Isarankura-Na-Ayudhya C, Prachayasittikul V (2014) QSAR study of H1N1 neuraminidase inhibitors from influenza a virus. Lett Drug Des Discov 11:420–427
Živković JV, Trutić NV, Veselinović JB, Nikolić GM, Veselinović AM (2015) Monte Carlo method based QSAR modeling of maleimide derivatives as glycogen synthase kinase-3β inhibitors. Comput Biol Med 64:276–282
Acknowledgements
A.A.T. and A.P.T thank the project LIFE-COMBASE contract (LIFE15 ENV/ES/000416) for financial support. I.Y. and T.E.-B. thank the Research Fund of Ankara University (Grant No: 12B3336002) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Nesměrák, K., Toropov, A.A., Toropova, A.P. et al. QSAR of antimycobacterial activity of benzoxazoles by optimal SMILES-based descriptors. Med Chem Res 26, 3203–3208 (2017). https://doi.org/10.1007/s00044-017-2013-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-2013-8