Abstract
We consider competing functional groups of tree species and develop a model of network response dynamics in order to measure the impacts of perturbations on the population distribution and diversity. The analysis of the equilibrium states relies on the connection between mean field game dynamics and replicator dynamics. We simulate our theoretical results from the data inventoried in French Guiana. Our results show that different types of disturbances modify the competitive interactions by affecting the evolutions of group densities. At the high regimes of disturbance, the canopy shade-intolerant species supplant the canopy shade-tolerant species. Tropical forest managers can thus take advantage of the competitive interactions between the functional groups to stimulate the abundance of marketable timber species. We also validate the hypothesis of maximum diversity at the intermediate disturbance levels.



Similar content being viewed by others
Notes
Despite the fact that intra-species interactions can be substantial, Doncaster (2009) showed that the inter-specific impacts are to be considered as of equal magnitude to that of the intra-specific impacts. Besides, the self-loops in the graph implicitly integrate the intra-group competition effects.
When \(\lim (C_{i,j})=1\), we implicitly account for the facilitative or mutual effects.
In detail, we have \(N_{i} \cdot \text {cov}\big (\sum _{i=1}^n N_{i} x_{i}, \sum _{j=1}^n N_{j} x_{j}\big ) = N_{i} \cdot \sum _{i=1}^n N_{i} \cdot \sum _{j=1}^n N_{j} \cdot \text {cov} \left( x_{i}, x_{j} \right) = \sum _{i=1}^n N_{i} \cdot \sum _{j=1}^n \left( 1 - N_{i} \right) \cdot N_{i} \cdot \text {cov} \left( x_{i}, x_{j} \right) = N_{i} \cdot \left( 1- N_{i} \right) \cdot \sum _{i=1}^n \sum _{j=1}^n N_{i} \cdot a_{j,i} = N_{i} \left( 1-N_{i}\right) C_{i,j}\).
According to this branch of game theory, the inter-type interactions must be sufficiently regular for the global phenomena to emerge.
The rest points of the replicator equation are those for which all payoff values are equal (Sigmund 2011). The population game with payoffs \(a_{j,i}\) is a full potential game if there is a continuous differential mapping \(M: \mathbb {R} \rightarrow \mathbb {R}\), that we can use as the inner product of the payoff vector and the direction of motion, such that \(\textit{d} M(N_{i})/ \textit{d}N_{i} = \textit{d}{N}_{i}/\textit{d}t\). The mean field game dynamics is positively correlated if \(\dot{N}_{i} \ne 0 \Rightarrow \langle a_{j,i}\left( N_{i}\right) , \dot{N}_{i} \rangle > 0\). On the contrary, the Euler stationarity would imply \(\dot{N}_{i} = 0\) (Franc et al. 2000).
Basal area is the area occupied by the cross-section of tree trunks and stems at their base.
The figures in brackets are negative values.
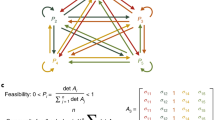
For example, \(C_{1,j}= N_{1} a_{1,1} + N_{2} a_{2,1} + N_{3} a_{3,1} + N_{4} a_{4,1} + N_{5} a_{5,1}\).
These trees may simply respond to logging because they are more demanding of light (Poorter et al. 2005).
In game theory, the decisions of agents are said to be strategic complements if they mutually increase their respective payoffs.
The same conclusion was empirically brought forward by Canham et al. (2006) within local neighborhoods, at the species level.
References
Allesina, S., & Levine, J. (2011). A competitive network theory of species diversity. Proceedings of the National Academy of Sciences, 108, 5638–5642.
Alvarez-Buylla, E., Garcia-Barrios, R., Lara-Moreno, C., & Martinez-Ramos, M. (1996). Demographic and genetic models in conservation biology: Applications and perspectives for tropical rain forest species. Annual Review of Ecology and Systematics, 27, 387–421.
Azaele, S., Pigolotti, S., Banavar, J., & Maritan, A. (2006). Dynamical evolution of ecosystems. Nature, 444, 926–928.
Barbosa, E., van Langevelde, F., Tomlinson, K., Carvalheiro, L., Kirkman, K., de Bie, S., & Prins, H. (2014). Tree species from different functional groups respond differently to environmental changes during establishment. Oecologia, 174, 1345–1357.
Bomze, I. (1983). Lotka–Volterra equation and replicator dynamics: A two-dimensional classification. Biological Cybernetics, 48, 201–211.
Buongiorno, J., Dahir, S., Lu, H.-C., & Lin, C. (1994). Tree size diversity and economic returns in uneven-aged forest stands. Forest Science, 40, 83–103.
Buongiorno, J., & Michie, B. (1980). A matrix model of uneven-aged forest management. Forest Science, 26, 609–625.
Buongiorno, J., Peyron, J.-L., Houllier, F., & Bruciamacchie, M. (1995). Growth and management of mixed-species, uneven-aged forests in the French Jura: Implications for economic returns and tree diversity. Forest Science, 41, 397–429.
Callaway, R. (2007). Positive interactions and interdependence in plant communities. New York: Springer.
Canham, C., Papaik, M., Uriarte, M., McWilliams, W., Jenkins, J., & Twery, M. (2006). Neighborhood analyses of canopy tree competition along environmental gradients in New England forests. Ecological Applications, 16, 540–554.
Chawanya, T., & Tokita, T. (2002). Large-dimensional replicator equations with anti-symmetric random interactions. Journal of the Physical Society of Japan, 71, 429–431.
Chazdon, R. (2008). Chance and determinism in tropical forest succession. In W. Carson & S. Schnitzer (Eds.), Tropical forest community ecology (pp. 384–408). Oxford: Wiley-Blackwell.
Chazdon, R., Finegan, B., Capers, R., Salgado-Negret, B., Casanoves, F., Boukili, V., & Norden, N. (2010). Composition and dynamics of functional groups of trees during tropical forest succession in northeastern Costa Rica. Biotropica, 42, 31–40.
Cheung, M.-W. (2014). Pairwise comparison dynamics for games with continuous strategy space. Journal of Economic Theory, 153, 344–375.
Cole, L., Bhagwat, S., & Willis, K. (2014). Recovery and resilience of tropical forests after disturbance. Nature Communications, 5, 1–7.
Connell, J. (1971). On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forests. In P. den Boer & G. Gradwell (Eds.), Dynamics of populations. Wageningen: Centre for Agricultural Publishing and Documentation.
Connor, E., & Simberloff, D. (1983). Interspecific competition and species co-occurrence patterns on islands: Null models and the evaluation of evidence. Oikos, 41, 455–465.
Dale, M. (2000). Spatial pattern analysis in plant ecology. Cambridge: Cambridge University Press.
Doncaster, C. (2009). Ecological equivalence: A realistic assumption for niche theory as a testable alternative to neutral theory. PLoS One, 4, 1–8.
El-Kassaby, Y., & Benowicz, A. (2000). Effects of commercial thinning on genetic, plant species and structural diversity in second growth Douglas-fir stands. Forest Genetics, 7, 193–203.
Fauset, S., Johnson, M., Gloor, M., Baker, T., Monteagudo, A., Brienen, R., Feldpausch, T., Lopez-Gonzalez, G., Malhi, Y., Ter Steege, H., Pitman, N., Baraloto, C., Engel, J., Pétronelli, P., Andrade, A., Camargo, J., Laurance, S., Laurance, W., Chave, J., Allie, E., Núñez Vargas, P., Terborgh, J., Ruokolainen, K., Silveira, M., Aymard, G., Arroyo, L., Bonal, D., Ramirez-Angulo, H., Araujo-Murakami, A., Neill, D., Hérault, B., Dourdain, A., Torres-Lezama, A., Marimon, B., Salomão, B., Comiskey, J., Réjou-Méchain, M., Toledo, M., Licona, J.-C., Alarcón, A., Prieto, A., Rudas, A., Van Der Meer, P., Killeen, T., Marimon Junior, B.-H., Poorter, L., Boot, R., Stergios, B., Vilanova Torre, E., Costa, F., Levis, C., Schietti, J., Souza, P., Groot, N., Arets, E., Chama Moscoso, V., Castro, W., Honorio Coronado, E., Peña-Claros, M., Stahl, C., Barroso, J., Talbot, J., Guimarães Vieira, I., Van Der Heijden, G., Thomas, R., Vos, V., Almeida, E., lvarez Davila, E., Aragão, L., Erwin, T., Morandi, P., Almeida de Oliveira, E., Valadão, M., Zagt, R., Van Der Hout, P., Alvarez Loayza, P., Pipoly, J., Wang, O., Alexiades, M., Cerón, C., Huamantupa-Chuquimaco, I., Di Fiore, A., Peacock, J., Pallqui Camacho, N., Umetsu, R., Barbosa De Camargo, P., Burnham, R., Herrera, R., Quesada, C., Stropp, J., Vieira, S., Steininger, M., Reynel Rodríguez, C., Restrepo, Z., Esquivel Muelbert, A., Lewis, S., Pickavance, G., & Phillips, O. (2015). Hyperdominance in Amazonian forest carbon cycling. Nature Communications, 6, 1–9.
Favrichon, V. (1998a). Apports d’un Modèle Démographique Plurispécifique pour l’Étude des Relations Diversité/Dynamique en Forêt Tropicale Guyanaise. Annals of Forest Science, 55, 655–669.
Favrichon, V. (1998b). Modeling the dynamics and species composition of a tropical mixed-species uneven-aged natural forest: Effects of alternative cutting regimes. Forest Science, 44, 113–124.
Fisher, D., & Reeves, R. (1995). Optimal strategies for random tournament games. Linear Algebra and its Applications, 217, 83–85.
Fort, H., & Inchausti, P. (2013). Tropical forests are non-equilibrium ecosystems governed by interspecific competition based on universal 1/6 niche width. PLoS One, 8(12), 1–9.
Fowler, N. (1981). Competition and coexistence in a North Carolina grassland. II. The effect of the experimental removal of species. Journal of Ecology, 69, 843–854.
Franc, A., Gourlet-Fleury, S., & Picard, N. (2000). Une Introduction à la Modélisation des Forêts Hétérogènes. Nancy: École Nationale du Génie Rural, des Eaux et des Forêts.
Franklin, J., Shugart, H., & Harmon, M. (1987). Tree death as an ecological process. Bioscience, 37, 550–556.
Harvey, C., Komar, O., Chazdon, R., Ferguson, B., Finegan, B., Griffith, D., Martinez-Ramos, M., Morales, H., Nigh, R., Soto-Pinto, L., van Breugel, M., & Wishnie, M. (2008). Integrating agricultural landscapes with biodiversity conservation in the Mesoamerican hotspot. Conservation Biology, 22, 8–15.
Hofbauer, J., & Sigmund, K. (1998). Evolutionary games and population dynamics. Cambridge: Cambridge University Press.
Hubbell, S. (2001). The unified neutral theory of biodiversity and biogeography. Princeton, NJ: Princeton University Press.
Hurlbert, S. (1990). Spatial distribution of the montane unicorn. Oikos, 58, 257–271.
Huston, M. (1979). A general hypothesis of species diversity. American Naturalist, 113, 81–101.
Institut National de la Statistique et des Études Économiques. (2004). La Forêt et le Bois en Guyane: le Cadre Institutionnel se Met en Place. Antiane, 61, 13–14.
Janzen, D. (1970). Herbivores and the number of tree species in tropical forest. American Naturalist, 104, 501–528.
Karev, G. (2006). Analytical models of forest dynamics in stable environment. In A. Burke (Ed.), New developments in ecology research. New York: Nova Science Publishers.
Laird, R., & Schamp, B. (2006). Competitive intransitivity promotes species coexistence. American Naturalist, 168, 182–193.
Laird, R., & Schamp, B. (2009). Species coexistence, intransitivity, and topological variation in competitive tournaments. Journal of Theoretical Biology, 256, 90–95.
Leslie, P. (1945). On the use of matrices in certain population mathematics. Biometrika, 33, 183–212.
Maître, H. (1982). Projet de Recherches Sylvicoles sur les Peuplements Naturels en Forêt Dense Guyanaise. Rapport Interne Centre Technique Forestier Tropical, Nogent sur Marne, France.
McDonald, E., Kruger, E., Riemenschneider, D., & Isebrands, J. (2002). Competitive status influences tree-growth responses to elevated \(\text{ CO }_{2}\) and \(\text{ O }_{3}\) in aggrading aspen stands. Functional Ecology, 16, 792–801.
Molino, J.-F., & Sabatier, D. (2001). Tree diversity in tropical rain forests: A validation of the intermediate disturbance hypothesis. Science, 294, 1702–1704.
Mulder, C., Bazeley-White, E., Dimitrakopoulos, P., Hector, A., Scherer-Lorenzen, M., & Schmid, B. (2004). Species evenness and productivity in experimental plant communities. Oikos, 107, 50–63.
Norden, N., Chazdon, R., Chao, A., Jiang, Y.-H., & Vilchez-Alvarado, B. (2009). Resilience of tropical rain forests: Tree community reassembly in secondary forests. Ecological Letters, 12, 385–394.
Phillips, O., Hall, P., Gentry, A., Sawyer, S., & Vasquez, R. (1994). Dynamics and species richness of tropical rain forests. Proceedings of the National Academy of Sciences, 91, 2805–2809.
Piélou, E. (1969). An introduction to mathematical ecology. New York: Wiley.
Poorter, L., Bongers, F., Sterck, F., & Woll, H. (2005). Beyond the regeneration phase: Differentiation of height-light trajectories among tropical tree species. Journal of Ecology, 93, 256–267.
Prévost, M., & Sabatier, D. (1993). Variations Spatiales de la Richesse et de la Diversité du Peuplement Arboré en Forêt Guyanaise. In Proceedings of Colloque de Phytogéographie Tropicale: Réalités et Perspectives, Muséum National d’Histoire Naturelle, Paris, France.
Sigmund, K. (2011). Introduction to evolutionary game theory. In Proceedings of symposia in applied mathematics, New Orleans, USA.
Silva Matos, D., Freckleton, R., & Watkinson, A. (1999). The role of density dependence in the population dynamics of a tropical palm. Ecology, 80, 2635–2650.
Tembine, H. (2011). Hybrid mean field game dynamics in large population. In American control conference, San Francisco, USA.
Tembine, H., Le Boudec, J.-Y., ElAzouzi, R., & Altman, E. (2009). From mean field interaction to evolutionary game dynamics. In Proceedings of the 7th international symposium on modeling and optimization in mobile, ad hoc and wireless networks, Seoul, South Korea.
Tilman, D. (1982). Resource competition and community structure. Princeton, NJ: Princeton University Press.
Ulrich, W., Soliveres, S., Kryszewski, W., Maestre, F., & Gotelli, N. (2014). Matrix models for quantifying competitive intransitivity from species abundance data. Oikos, 123, 1057–1070.
Usher, M. (1976). Extensions to models, used in renewable resource management, which incorporate an arbitrary structure. Journal of Environmental Management, 4, 123–140.
Vanclay, J. (1991). Aggregating tree species to develop diameter increment equations for tropical rainforests. Forest Ecology and Management, 42, 143–168.
Vanclay, J. (1995). Growth models for tropical forests: A synthesis of models and methods. Forest Science, 41, 7–42.
Vandermeer, J., & Yitbarek, S. (2012). Self-organized spatial pattern determines biodiversity in spatial competition. Journal of Theoretical Biology, 300, 48–56.
Volkov, I., Banavar, J., Hubbell, S., & Maritan, A. (2003). Neutral theory and relative species abundance in ecology. Letters to Nature, 424, 1035–1037.
Zhang, J., Huang, S., & He, F. (2015). Half-century evidence from western Canada shows forest dynamics are primarily driven by competition followed by climate. Proceedings of the National Academy of Sciences, 112, 4009–4014.
Acknowledgments
This work was financially supported by the French National Research Agency through the Laboratory of Excellence ARBRE, a part of the Investments for the Future Program (ANR 11 – LABX-0002-01). It was also supported by the French National Forestry Office through the Forests for Tomorrow Chair. We would like to thank the CIRAD Research Center for allowing us to access to the Paracou database, and wish to acknowledge the support of Eric Marcon (Agro ParisTech) and Bruno Ferry (Agro ParisTech). We are also grateful to the editor and the two anonymous referees for their thorough comments and suggestions, which significantly contributed to improving the quality of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dragicevic, A.Z. Functional diversity from network response dynamics. J Bioecon 18, 1–15 (2016). https://doi.org/10.1007/s10818-015-9206-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10818-015-9206-3