Abstract
Aim
The aim of this study is the evaluation effect of nanoliposome-loaded Mito-Tempo on sperm parameters during human sperm cryopreservation.
Methods
Semen samples of 50 Asthenoteratozoospermia men (random) were collected. Sperm parameters were analyzed based on World Health Organization (WHO, 2010) criteria (2021) and each sample was divided into 5 groups (E1–E5). E1 (control group): the sperm was cryopreserved without nanoliposome, and Mito-Tempo. E2: sperm cryopreservation with Mito-Tempo-loaded nanoliposome (Mito-Tempo 0.1 mM) + freezing medium. E3: sperm cryopreservation with Mito-Tempo-loaded nanoliposome (Mito-Tempo 0.2 mM) + freezing medium. E4: in this group, the cryopreservation sperm with Mito-Tempo 0.3 mM + freezing medium. E5: the cryopreservation sperm with Mito-Tempo 0.2 mM + freezing medium.
Results
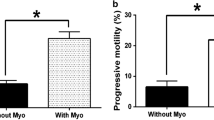
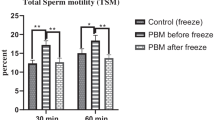
The result of this study indicated that sperm parameters and total antioxidant capacity (TAC) significantly increase in E3 and E4 groups, compared to E1, E2, and E5 groups respectively (P < 0.05). The percentage of abnormal morphology, DNA fragmentation index (DFI), malondialdehyde (MDA), and the levels of ROS significantly decrease in E3 and E4 groups, compared to E1, E2, and E5 groups (P < 0.05). In addition, the sperm parameters and stress oxidative factors significantly improve in E3 group compared to other groups (P < 0.05).
Conclusions
In conclusion, the combination of Mito-Tempo with nanoliposome due to its ability to cooperate with lipid layers may lead to significant performance in reducing oxidative stress damage and increasing the quality of sperm parameters.










Similar content being viewed by others
Data availability
The primary data for this study is available from the authors upon direct request.
References
Paoli D, Pelloni M, Lenzi A, Lombardo F. Cryopreservation of sperm: effects on chromatin and strategies to prevent them. Adv Exp Med Biol. 2019;1166:149–67. https://doi.org/10.1007/978-3-030-21664-1_9
Le MT, Nguyen TTT, Nguyen TT, Nguyen TV, Nguyen TAT, Nguyen QHV, et al. Does conventional freezing affect sperm DNA fragmentation? Clin Exp Reprod Med. 2019;46(2):67–75. https://doi.org/10.5653/cerm.2019.46.2.67
Aitken RJ. Impact of oxidative stress on male and female germ cells: implications for fertility. Reproduction. 2020;159(4):R189-r201. https://doi.org/10.1530/rep-19-0452.
Cheng Q, Li L, Jiang M, Liu B, Xian Y, Liu S, et al. Extend the survival of human sperm in vitro in non-freezing conditions: damage mechanisms, preservation technologies, and clinical applications. Cells. 2022;11(18):2845.
Agarwal A, Farkouh Aa, Saleh R, Hamoda TA-AA-M, Harraz AM, Kavoussi P, et al. Controversy and consensus on indications for sperm DNA fragmentation testing in male infertility: a global survey, current guidelines, and expert recommendations. World J Men’s Health. 2023;41(3):575.
Motlagh MK, Sharafi M, Zhandi M, Mohammadi-Sangcheshmeh A, Shakeri M, Soleimani M, et al. Antioxidant effect of rosemary (Rosmarinus officinalis L.) extract in soybean lecithin-based semen extender following freeze–thawing process of ram sperm. Cryobiology. 2014;69(2):217–22.
Lu X, Zhang Y, Bai H, Liu J, Li J, Wu B. Mitochondria-targeted antioxidant MitoTEMPO improves the post-thaw sperm quality. Cryobiology. 2018;80:26–9.
Jannatifar R, Asa E, Sahraei SS, Verdi A, Piroozmanesh H. N-acetyl‐l‐cysteine and alpha lipoic acid are protective supplement on human sperm parameters in cryopreservation of asthenoteratozoospermia patients. Andrologia. 2022;54(11):e14612.
Zhang X, Lu X, Li J, Xia Q, Gao J, Wu B. Mito-Tempo alleviates cryodamage by regulating intracellular oxidative metabolism in spermatozoa from asthenozoospermic patients. Cryobiology. 2019;91:18–22.
Wang P-F, Xie K, Cao Y-X, Zhang A. Hepatoprotective effect of mitochondria-targeted antioxidant mito-TEMPO against lipopolysaccharide-induced liver injury in mouse. Mediators of inflammation. 2022;2022.
SAYLAN A, DIRAMALI M. The effects of Mito-TEMPO, a mitochondria targeted antioxidant, on frozen human sperm parameters. Turkish J Health Sport Volume. 2023;4(1):1.
Jannatifar R, Parivar K, Roodbari NH, Nasr-Esfahani MH. Effects of N-acetyl-cysteine supplementation on sperm quality, chromatin integrity and level of oxidative stress in infertile men. Reproductive Biology Endocrinol. 2019;17(1):1–9.
Najafi A, Kia HD, Mehdipour M, Hamishehkar H, Álvarez-Rodríguez M. Effect of quercetin loaded liposomes or nanostructured lipid carrier (NLC) on post-thawed sperm quality and fertility of rooster sperm. Theriogenology. 2020;152:122–8.
Bhaskar S, Tian F, Stoeger T, Kreyling W, de la Fuente JM, Grazú V, et al. Multifunctional nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: perspectives on tracking and neuroimaging. Part Fibre Toxicol. 2010;7(1):1–25.
Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MdP, Acosta-Torres LS, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018;16(1):1–33.
Liu R, Luo C, Pang Z, Zhang J, Ruan S, Wu M, et al. Advances of nanoparticles as drug delivery systems for disease diagnosis and treatment. Chin Chem Lett. 2023;34(2):107518.
Iqbal MA, Md S, Sahni JK, Baboota S, Dang S, Ali J. Nanostructured lipid carriers system: recent advances in drug delivery. J Drug Target. 2012;20(10):813–30.
Garg N, Ahmad FJ. Epigallocatechin-3-gallate-loaded nanocarriers for health benefits. Nanomedicine for Bioactives: Healthcare applications. 2020:393–411.
Huang S-L. Liposomes in ultrasonic drug and gene delivery. Adv Drug Deliv Rev. 2008;60(10):1167–76.
Eloy JO, de Souza MC, Petrilli R, Barcellos JPA, Lee RJ, Marchetti JM. Liposomes as carriers of hydrophilic small molecule drugs: strategies to enhance encapsulation and delivery. Colloids Surf B. 2014;123:345–63.
Martínez-Ballesta M, Gil-Izquierdo Á, García-Viguera C, Domínguez-Perles R. Nanoparticles and controlled delivery for bioactive compounds: outlining challenges for new smart-foods for health. Foods. 2018;7(5):72.
Organization WH. WHO laboratory manual for the examination and processing of human semen. World Health Organization; 2021.
Ziarati N, Topraggaleh TR, Rahimizadeh P, Montazeri L, Maroufizadeh S, Gilani MAS, et al. Micro-quantity straw as a carrier for cryopreservation of oligozoospermic semen samples: effects of storage times and cryoprotectant. Cryobiology. 2019;86:65–70.
Quintela A, Oliveira I, Souza A, Gusmão A, Silva A. Water-induced hypo-osmotic test for the evaluation of canine sperm membrane integrity. Anim Reprod (AR). 2018;7(2):70–4.
Kheirollahi-Kouhestani M, Razavi S, Tavalaee M, Deemeh M, Mardani M, Moshtaghian J, et al. Selection of sperm based on combined density gradient and Zeta method may improve ICSI outcome. Hum Reprod. 2009;24(10):2409–16.
Keller A, Mohamed A, Dröse S, Brandt U, Fleming I, Brandes RP. Analysis of dichlorodihydrofluorescein and dihydrocalcein as probes for the detection of intracellular reactive oxygen species. Free Radic Res. 2004;38(12):1257–67.
Marchetti C, Jouy N, Leroy-Martin B, Defossez A, Formstecher P, Marchetti P. Comparison of four fluorochromes for the detection of the inner mitochondrial membrane potential in human spermatozoa and their correlation with sperm motility. Hum Reprod. 2004;19(10):2267–76.
Zhu Z, Li R, Lv Y, Zeng W. Melatonin protects rabbit spermatozoa from cryo-damage via decreasing oxidative stress. Cryobiology. 2019;88:1–8.
Hassan MA, Khalil WA, Abdelnour SA, Aman RM. Supplementation of alpha-lipoic acid-loaded nanoliposomes in semen extender improves freezability of buffalo spermatozoa. Sci Rep. 2022;12(1):22464.
Naidoo S. Studies on factors influencing viability after cryopreservation of excised zygotic embryos from recalcitrant seeds of two amaryllid species 2010.
Lembo D, Donalisio M, Civra A, Argenziano M, Cavalli R. Nanomedicine formulations for the delivery of antiviral drugs: a promising solution for the treatment of viral infections. Expert Opin Drug Deliv. 2018;15(1):93–114.
Pateiro M, Gómez B, Munekata PE, Barba FJ, Putnik P, Kovačević DB, et al. Nanoencapsulation of promising bioactive compounds to improve their absorption, stability, functionality and the appearance of the final food products. Molecules. 2021;26(6):1547.
Alfei S. Nanotechnology applications to improve solubility of bioactive constituents of foods for health-promoting purposes. Nano-food Engineering: Volume One. 2020:189–257.
Wijekoon MJO, Mahmood K, Ariffin F, Nafchi AM, Zulkurnain M. Recent advances in encapsulation of fat-soluble vitamins using polysaccharides, proteins, and lipids: a review on delivery systems, formulation, and industrial applications. Int J Biol Macromol. 2023:124539.
Tabibiazar M, Mohammadifar MA, Roufegarinejad L, Ghorbani M, Hashemi M, Hamishehkar H. Improvement in dispersibility, stability and antioxidant activity of resveratrol using a colloidal nanodispersion of BSA-resveratrol. Food Bioscience. 2019;27:46–53.
Zielonka J, Joseph J, Sikora A, Hardy M, Ouari O, Vasquez-Vivar J, et al. Mitochondria-targeted triphenylphosphonium-based compounds: syntheses, mechanisms of action, and therapeutic and diagnostic applications. Chem Rev. 2017;117(15):10043–120.
Atayik MC, Çakatay U. Mitochondria-targeted senotherapeutic interventions. Biogerontology. 2022;23(4):401–23.
Partyka A, Niżański W. Supplementation of avian semen extenders with antioxidants to improve semen quality—is it an effective strategy? Antioxidants. 2021;10(12):1927.
Shi L, Shi J, Feng J, Zhang P, Ren Y. Proteomic analysis reveals the potential positive effects of Mito-TEMPO on ram sperm motility and fertility during cryopreservation. Theriogenology. 2023;205:27–39.
Zidni I, Lee HB, Yoon JH, Park JY, Oh YD, Jang HS, et al. Effect of antioxidants in cryopreservation media on spotted halibut (Verasfer Variegatus) sperm quality during cryopreservation. Aquaculture. 2022;557:738351.
Vaughn AK. Mitochondrial formate production and its impact on embryonic cranial tissue development in the mouse. The University of Texas at Austin; 2019.
Lemma A. Effect of cryopreservation on sperm quality and fertility. Artif Insemin farm Anim. 2011;12:191–216.
Dadras H, Golpour A, Rahi D, Lieskovská J, Dzyuba V, Gazo I, et al. Cryopreservation of sterlet, Acipenser ruthenus spermatozoa: evaluation of quality parameters and fine ultrastructure. Front Mar Sci. 2022;9:783278.
Kumar A, Kumar Ghosh S, Katiyar R, Gemeda AE, Rautela R, Bisla A, et al. Supplementation of Mito TEMPO and acetovanillone in semen extender improves freezability of buffalo spermatozoa. Andrology. 2022;10(4):775–88.
Asadzadeh N, Abdollahi Z, Esmaeilkhanian S, Masoudi R. Fertility and flow cytometry evaluations of ram frozen semen in plant-based extender supplemented with Mito-TEMPO. Anim Reprod Sci. 2021;233:106836.
Zarei F, Kia HD, Masoudi R, Moghaddam G, Ebrahimi M. Supplementation of ram’s semen extender with Mito-TEMPO I: improvement in quality parameters and reproductive performance of cooled-stored semen. Cryobiology. 2021;98:215–8.
Aitken RJ. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol Reprod Dev. 2017;84(10):1039–52.
Liu Y, Perumal E, Bi X, Wang Y, Ding W. Potential mechanisms of uremic muscle wasting and the protective role of the mitochondria-targeted antioxidant Mito-TEMPO. Int Urol Nephrol. 2020;52:1551–61.
Karthikeyan A, Senthil N, Min T, Nanocurcumin. A promising candidate for therapeutic applications. Front Pharmacol. 2020;11:487.
Ríos J-L, Giner RM, Marín M, Recio MC. A pharmacological update of ellagic acid. Planta Med. 2018;84(15):1068–93.
Du Plessis SS, Agarwal A, Halabi J, Tvrda E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J Assist Reprod Genet. 2015;32:509–20.
Jannatifar R, Piroozmanesh H, Jannatifar Z. Effect of N-acetylcysteine on human sperm parameters and DNA damage in frozen-thawed sperm samples of asthenozoospermic men. Qom Univ Med Sci J. 2021;14(12):32–40.
Acknowledgements
This study was supported by IVF Unit of Infertility, and Research Center of the ACECR, Qom.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of the Mashhad.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jannatifar, R., Piroozmanesh, H., Sahraei, S.S. et al. The evaluation effect of nanoliposome-loaded Mito-Tempo on sperm parameters during human sperm cryopreservation. J Assist Reprod Genet (2024). https://doi.org/10.1007/s10815-024-03132-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10815-024-03132-7