Abstract
Purpose
To evaluate the decline in transferable embryos in preimplantation genetic testing for aneuploidy (PGT-A) cycles due to (a) non-biopsable blastocyst quality, (b) failure of genetic analysis, (c) diagnosis of uniform numerical or structural chromosomal aberrations, and/or (d) chromosomal aberrations in mosaic constitution.
Methods
This retrospective multicenter study comprised outcomes of 1562 blastocysts originating from 363 controlled ovarian stimulation cycles, respectively, 226 IVF couples in the period between January 2016 and December 2018. Inclusion criteria were PGT-A cycles with trophectoderm biopsy (TB) and next generation sequencing (NGS).
Results
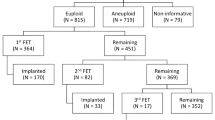
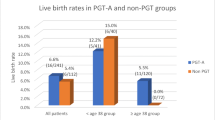
Out of 1562 blastocysts, 25.8% were lost due to non-biopsable and/or non-freezable embryo quality. In 10.3% of all biopsied blastocysts, genetic analysis failed. After exclusion of embryos with uniform or chromosomal aberrations in mosaic, only 18.1% of those originally yielded remained as diagnosed euploid embryos suitable for transfer. This translates into 50.4% of patients and 57.6% of stimulated cycles with no euploid embryo left for transfer. The risk that no transfer can take place rose significantly with a lower number of oocytes and with increasing maternal age. The chance for at least one euploid blastocyst/cycle in advanced maternal age (AMA)-patients was 33.3% compared to 52.1% in recurrent miscarriage (RM), 59.8% in recurrent implantation failure (RIF), and 60.0% in severe male factor (SMF).
Conclusions
The present study demonstrates that PGT-A is accompanied by high embryo drop-out rates. IVF-practitioners should be aware that their patients run a high risk of ending up without any embryo suitable for transfer after (several) stimulation cycles, especially in AMA patients. Patients should be informed in detail about the frequency of inconclusive or mosaic results, with the associated risk of not having an euploid embryo available for transfer after PGT-A, as well as the high cost involved in this type of testing.




Similar content being viewed by others
Data availability
The data presented in this manuscript are not publicly available due to data protection restrictions.
References
Munne S. Status of preimplantation genetic testing and embryo selection. Reprod BioMed Online. 2018;37:393–6.
Twisk M, Mastenbroek S, van Wely M, Heineman MJ, Van der Veen F, Repping S. Preimplantation genetic screening for abnormal number of chromosomes (aneuploidies) in in vitro fertilisation or intracytoplasmic sperm injection. Cochrane Database Syst Rev. 2006;1:CD005291.
Cornelisse S, Zagers M, Kostova E, Fleischer K, van Wely M, Mastenbroek S. Preimplantation genetic testing for aneuploidies (abnormal number of chromosomes) in in vitro fertilisation. Cochrane Database Syst Rev. 2020;9:CD005291.
Scott RT Jr, Upham KM, Forman EJ, Zhao T, Treff NR. Cleavage-stage biopsy significantly impairs human embryonic implantation potential while blastocyst biopsy does not: a randomized and paired clinical trial. Fertil Steril. 2013;100:624–30.
Magli MC, Gianaroli L, Fortini D, Ferraretti AP, Munné S. Impact of blastomere biopsy and cryopreservation techniques on human embryo viability. Hum Reprod. 1999;14:770–3.
Fiorentino F, Biricik A, Bono S, Spizzichino L, Cotroneo E, Cottone G, Kokocinski F, Michel CE. Development and validation of a next-generation sequencing-based protocol for 24-chromosome aneuploidy screening of embryos. Fertil Steril. 2014;101:1375–82.
Capalbo A, Rienzi L. Mosaicism between trophectoderm and inner cell mass. Fertil Steril. 2017;107:1098–106.
Lai HH, Chuang TH, Wong LK, Lee MJ, Hsieh CL, Wang HL, Chen SU. Identification of mosaic and segmental aneuploidies by next-generation sequencing in preimplantation genetic screening can improve clinical outcomes compared to array-comparative genomic hybridization. Mol Cytogenet. 2017;10:14.
Munné S, Kaplan B, Frattarelli JL, Child T, Nakhuda G, Shamma FN, Silverberg K, Kalista T, Handyside AH, Katz-Jaffe M, Wells D, Gordon T, Stock-Myer S, Willman S, STAR Study Group. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112:1071–79.e7.
Yan J, Qin Y, Zhao H, Sun Y, Gong F, Li R, Sun X, Ling X, Li H, Hao C, Tan J, Yang J, Zhu Y, Liu F, Chen D, Wei D, Lu J, Ni T, Zhou W, et al. Live birth with or without preimplantation genetic testing for aneuploidy. N Engl J Med. 2021;385:2047–58. https://doi.org/10.1056/NEJMoa2103613.
Orvieto R, Gleicher N. Preimplantation genetic testing for aneuploidy (PGT-A)—finally revealed. J Assist Reprod Genet. 2020;37:669–72.
Schattman GL. Preimplantation genetic testing for aneuploidy: it’s déjà vu all over again! Fertil Steril. 2019;112:1046–7.
Gleicher N, Albertini DF, Barad DH, Homer H, Modi D, Murtinger M, Patrizio P, Orvieto R, Takahashi S, Weghofer A, Ziebe S, Noyes N, International Do No Harm Group in IVF (IDNHG-IVF). The 2019 PGDIS position statement on transfer of mosaic embryos within a context of new information on PGT-A. Reprod Biol Endocrinol. 2020;18:57.
Homer HA. Preimplantation genetic testing for aneuploidy (PGT-A): the biology, the technology and the clinical outcomes. Aust N Z J Obstet Gynaecol. 2019;59:317–24.
Zhang YX, Chen JJ, Nabu S, Yeung QSY, Li Y, Tan JH, Suksalak W, Chanchamroen S, Quangkananurug W, Wong PS, Chung JPW, Choy KW. The pregnancy outcome of mosaic embryo transfer: a prospective multicenter study and meta-analysis. Genes (Basel). 2020;11:973.
Wirleitner B, Okhowat J, Vištejnová L, Králíčková M, Karlíková M, Vanderzwalmen P, Ectors F, Hradecký L, Schuff M, Murtinger M. Relationship between follicular volume and oocyte competence, blastocyst development and live-birth rate: optimal follicle size for oocyte retrieval. Ultrasound Obstet Gynecol. 2018;51:118–25.
Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11:307–11.
Wirleitner B, Vanderzwalmen P, Stecher A, Murtinger M. Aseptic vitrification of blastocysts after trophectoderm (TE) biopsy. Reprod BioMed Online. 2018;36(Suppl 1):e13.
Chuang TH, Chang YP, Lee MJ, Wang HL, Lai HH, Chen SU. The incidence of mosaicism for individual chromosome in human blastocysts is correlated with chromosome length. Front Genet. 2021;11:565348.
Cimadomo D, Rienzi L, Romanelli V, Alviggi E, Levi-Setti PE, Albani E, Dusi L, Papini L, Livi C, Benini F, Smeraldi A, Patassini C, Ubaldi FM, Capalbo A. Inconclusive chromosomal assessment after blastocyst biopsy: prevalence, causative factors and outcomes after re-biopsy and re-vitrification. A multicenter experience. Hum Reprod. 2018;33:1839–46.
Bundesministerium für Digitalisierung und Wirtschaftsstandort. Bundesrecht konsolidiert: Gesamte Rechtsvorschrift für Fortpflanzungsmedizingesetz, Fassung vom 19.11.2022. Available from: https://www.ris.bka.gv.at/GeltendeFassung.wxe?Abfrage=Bundesnormen&Gesetzesnummer=10003046 Accessed 20 Nov 2022.
Gleicher N, Metzger J, Croft G, Kushnir VA, Albertini DF, Barad DH. A single trophectoderm biopsy at blastocyst stage is mathematically unable to determine embryo ploidy accurately enough for clinical use. Reprod Biol Endocrinol. 2017;15:33.
Yang H, DeWan AT, Desai MM, Vermund SH. Preimplantation genetic testing for aneuploidy: challenges in clinical practice. Hum Genomics. 2022;16:69.
Takahashi S, Johnston J, Patrizio P. Lessons from the premature adoption of preimplantation embryo testing. Genet Med. 2019;21:1038–40.
ESHRE Working Group on Chromosomal Mosaicism, De Rycke M, Capalbo A, Coonen E, Coticchio G, Fiorentino F, Goossens V, Mcheik S, Rubio C, Sermon K, Sfontouris I, Spits C, Vermeesch JR, Vermeulen N, Wells D, Zambelli F, Kakourou G. ESHRE survey results and good practice recommendations on managing chromosomal mosaicism. sHum Reprod Open. 2022;2022:hoac044. https://doi.org/10.1093/hropen/hoac044.
Cram DS, Leigh D, Handyside A, Rechitsky L, Xu K, Harton G, Grifo J, Rubio C, Fragouli E, Kahraman S, Forman E, Katz-Jaffe M, Tempest H, Thornhill A, Strom C, Escudero T, Qiao J, Munne S, Simpson JL, Kuliev A. PGDIS position statement on the transfer of mosaic embryos 2019. Reprod BioMed Online. 2019;39(Suppl 1):e1–4.
Xie P, Liu P, Zhang S, Cheng D, Chen D, Tan YQ, Hu L, Qiu Y, Zhou S, Ou-Yang Q, Luo K, Lu G, Zhang S, Gong F, Lin G. Segmental aneuploidies with 1 Mb resolution in human preimplantation blastocysts. Genet Med. 2022;24:2285–95. https://doi.org/10.1016/j.gim.2022.08.008.
McCoy RC. Mosaicism in preimplantation human embryos: when chromosomal abnormalities are the norm. Trends Genet. 2017;33:448–63.
Abhari S, Kawwass JF. Pregnancy and neonatal outcomes after transfer of mosaic embryos: a review. J Clin Med. 2021;10:1369.
Shahbazi MN, Wang T, Tao X, Weatherbee BAT, Sun L, Zhan Y, Keller L, Smith GD, Pellicer A, Scott RT Jr, Seli E, Zernicka-Goetz M. Developmental potential of aneuploid human embryos cultured beyond implantation. Nat Commun. 2020;11:3987.
Singla S, Iwamoto-Stohl LK, Zhu M, Zernicka-Goetz M. Autophagy-mediated apoptosis eliminates aneuploid cells in a mouse model of chromosome mosaicism. Nat Commun. 2020;11:2958.
Yang M, Rito T, Metzger J, Naftaly J, Soman R, Hu J, Albertini DF, Barad DH, Brivanlou AH, Gleicher N. Depletion of aneuploid cells in human embryos and gastruloids. Nat Cell Biol. 2021;23:314–21.
Gleicher N, Kushnir VA, Barad DH. How PGS/PGT-A laboratories succeeded in losing all credibility. Reprod BioMed Online. 2018;37:242–5.
Murtinger M, Wirleitner B, Schuff M. Scoring of mosaic embryos after preimplantation genetic testing: a rollercoaster ride between fear, hope and embryo wastage. Reprod BioMed Online. 2018;37:120–1.
Braude P. The emperor still looks naked. Reprod BioMed Online. 2018;37:133–5.
Wirleitner B, Schuff M, Stecher A, Murtinger M, Vanderzwalmen P. Pregnancy and birth outcomes following fresh or vitrified embryo transfer according to blastocyst morphology and expansion stage, and culturing strategy for delayed development. Hum Reprod. 2016;31:1685–95.
Gleicher N, Barad DH, Ben-Rafael Z, Glujovsky D, Mochizuki L, Modi D, Murtinger M, Patrizio P, Orvieto R, Takahashi S, Weghofer A, Ziebe S, International Do No Harm Group in IVF (IDNHG-IVF). Commentary on two recently published formal guidelines on management of “mosaic” embryos after preimplantation genetic testing for aneuploidy (PGT-A). Reprod Biol Endocrinol. 2021;19:23. https://doi.org/10.1186/s12958-021-00716-1.
Murtinger M, Schuff M, Wirleitner B, Miglar S, Spitzer D. Comment on the recent PGDIS position statement on the transfer of mosaic embryos 2021. J Assist Reprod Genet. 2022; https://doi.org/10.1007/s10815-022-02620-y.
Lin J, Vitek W, Scott EL. Order from chaos: a case report of a healthy live birth from a genetically “chaotic” embryo. F S Rep. 2022;3:301–4. https://doi.org/10.1016/j.xfre.2022.10.003.
Listorti I, Ronsini C, Greco PF, Victor A, Barnes F, Zouves C, Spinella F, Viotti M. Two clinical case reports of embryonic mosaicism identified with PGT-A persisting during pregnancy as true fetal mosaicism. Hum Reprod. 2023:deac263. https://doi.org/10.1093/humrep/deac263.
Gleicher N, Patrizio P, Mochizuki L, Barad DH. Previously reported and here added cases demonstrate euploid pregnancies followed by PGT-A as “mosaic” as well as “aneuploid” designated embryos. Reprod Biol Endocrinol. 2023;21:25.
Schlade-Bartusiak K, Strong E, Zhu O, Mackie J, Salema D, Volodarsky M, Roberts J, Steinraths M. Mosaic embryo transfer-first report of a live born with nonmosaic partial aneuploidy and uniparental disomy 15. F S Rep. 2022;3:192–7. https://doi.org/10.1016/j.xfre.2022.05.003.
Franasiak JM. Mosaic embryo transfer: a cautionary tale. F S Rep. 2022;3:179–80. https://doi.org/10.1016/j.xfre.2022.07.005.
Tocci A. The unknown human trophectoderm: implication for biopsy at the blastocyst stage. J Assist Reprod Genet. 2020;37:2699–711.
Yang Z, Liu J, Collins GS, Salem SA, Liu X, Lyle SS, Peck AC, Sills ES, Salem RD. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Mol Cytogenet. 2012;5:24.
Konstantinidis M, Prates R, Goodall NN, Fischer J, Tecson V, Lemma T, Chu B, Jordan A, Armenti E, Wells D, Munné S. Live births following Karyomapping of human blastocysts: experience from clinical application of the method. Reprod BioMed Online. 2015;31:394–403.
Osman EK, Neal SA, Tiegs AW, Hanson BM, Kim JG, Franasiak JM, Scott RT Jr. Consistency in rates of diagnosis of embryonic mosaicism, segmental abnormalities, and “no call” results among experienced embryologists performing preimplantation genetic testing for aneuploidy. F S Rep. 2020;1:119–24.
Lee H, McCulloh DH, Olivares R, Goldstein-Tufaro A, McCaffrey C, Grifo J. Live births after transfer of rebiopsy and revitrification of blastocyst that had “no diagnosis” following trophectoderm biopsy. Fertil Steril. 2016;106:e164.
Brower M, Hill D, Danzer H, Surrey M, Ghadir S, Chang W, Wambach C, Alexander C, Barritt J. “No diagnosis” embryos after PGS should not be discarded: rebiopsy and reanalysis demonstrate the majority are euploid. Fertil Steril. 2014;102:e31.
Kaing A, Kroener L, Brower M, Hill D, Danzer H, Barritt J. Rebiopsy and preimplanation genetic screening (PGS) reanalysis demonstrate the majority of originally “no diagnosis” embryos are euploid with comparable pregnancy rates. Fertil Steril. 2015;104:e277.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wirleitner, B., Hrubá, M., Schuff, M. et al. Embryo drop-out rates in preimplantation genetic testing for aneuploidy (PGT-A): a retrospective data analysis from the DoLoRes study. J Assist Reprod Genet 41, 193–203 (2024). https://doi.org/10.1007/s10815-023-02976-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-02976-9