Abstract
Research question
To investigate differences in reproductive outcomes among IVF patients with lean compared to obese polycystic ovarian syndrome (PCOS) phenotypes.
Design
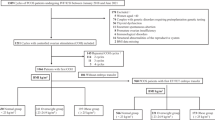
A retrospective cohort study of patients with PCOS who underwent IVF in a single, academically affiliated infertility center in the USA between December 2014 and July 2020. The diagnosis of PCOS was assigned based on Rotterdam criteria. Patients were designated as lean (< 25) or overweight/obese (≥ 25) PCOS phenotype based on BMI (kg/m2) at cycle start. Baseline clinical and endocrinologic laboratory panel, cycle characteristics, and reproductive outcomes were analyzed. The cumulative live birth rate included up to 6 consecutives cycles. A Cox proportional hazards model and Kaplan–Meier curve for estimating live birth rates were used to compare the two phenotypes.
Results
A total of 1395 patients who underwent 2348 IVF cycles were included. The mean (SD) BMI was 22.7 (2.4) in the lean and 33.8 (6.0) in the obese group (p < 0.001). A number of endocrinological parameters were similar between lean and obese phenotypes: total testosterone 30.8 ng/dl (19.5) vs 34.1 (21.9), p > 0.02 and pre-cycle hemoglobin A1C 5.33% (0.38) vs 5.51% (0.51) p > 0.001, respectively. The CLBR was higher in those with a lean PCOS phenotype: 61.7% (373/604) vs 54.0% (764/1414) respectively. Miscarriage rates were significantly higher for O-PCOS patients (19.7% (214/1084) vs 14.5% (82/563) p < 0.001) and the rate of aneuploids was similar (43.5%, 43.8%, p = 0.8). A Kaplan–Meier curve estimating the proportion of patients with a live birth was higher in the lean group (log-rank test p = 0.013). After adjusting for potential confounders, the lean phenotype was associated with an increased hazard ratio for live birth: HR = 1.38 p < 0.001.
Conclusions
Lean PCOS phenotype is associated with a significantly higher CLBR compared to their obese counterparts. Miscarriage rates were significantly higher among obese patients, despite comparable pre-cycle HBA1C and similar aneuploidy rates in patients who underwent PGT-A.



Similar content being viewed by others
Data availability
Supplemental data included.
Code availability
Not applicable.
References
Argenta P, et al. Hormone receptor expression patterns in the endometrium of asymptomatic morbidly obese women before and after bariatric surgery. Gynecol Oncol. 2014;133(1):78–82.
ASRM. Medicine. Obesity and reproduction: a committee opinion. Fertil Steril. 2021;116(5):1266–85.
Bell GA, et al. Maternal polycystic ovarian syndrome and early offspring development. Hum Reprod. 2018;33(7):1307–15.
Bellver J, et al. Endometrial gene expression in the window of implantation is altered in obese women especially in association with polycystic ovary syndrome. Fertil Steril. 2011;95(7):2335–41 (2341.e2331-2338).
Bergin K, et al. The use of propensity score matching to assess the benefit of the endometrial receptivity analysis in frozen embryo transfers. Fertil Steril. 2021;116(2):396–403.
Bozdag G, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31(12):2841–55.
Brewer CJ, Balen AH. The adverse effects of obesity on conception and implantation. Reproduction. 2010;140(3):347–64.
Chatzakis C, et al. Different pregnancy outcomes according to the polycystic ovary syndrome diagnostic criteria: a systematic review and meta-analysis of 79 studies. Fertil Steril. 2022;117(4):854–81.
Clark AM, et al. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Hum Reprod. 1995;10(10):2705–12.
Comstock IA, et al. Does an increased body mass index affect endometrial gene expression patterns in infertile patients? A functional genomics analysis. Fertil Steril. 2017;107(3):740-748.e742.
Conway G, et al. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol. 2014;171(4):P1-29.
Diamanti-Kandarakis E. Role of obesity and adiposity in polycystic ovary syndrome. Int J Obes (Lond). 2007;31 Suppl 2:S8-13 (discussion S31-12).
Einarsson S, et al. Weight reduction intervention for obese infertile women prior to IVF: a randomized controlled trial. Hum Reprod. 2017;32(8):1621–30.
Fedorcsák P, et al. Impact of overweight and underweight on assisted reproduction treatment. Hum Reprod. 2004;19(11):2523–8.
Gambineri A, et al. Obesity and the polycystic ovary syndrome. Int J Obes Relat Metab Disord. 2002;26(7):883–96.
Goldman RH, et al. The combined impact of maternal age and body mass index on cumulative live birth following in vitro fertilization. Am J Obstet Gynecol. 2019;221(6):617.e611-617.e613.
Goyal M, Dawood AS. Debates regarding lean patients with polycystic ovary syndrome: a narrative review. J Hum Reprod Sci. 2017;10(3):154–61.
Grodstein F, et al. Body mass index and ovulatory infertility. Epidemiology. 1994;5(2):247–50.
Hughes LM, et al. Maternal body mass index is not associated with increased rates of maternal embryonic aneuploidy. Fertil Steril. 2022;117(4):783–9.
Jungheim ES, et al. IVF outcomes in obese donor oocyte recipients: a systematic review and meta-analysis. Hum Reprod. 2013;28(10):2720–7.
Kakoly NS, et al. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta-regression. Hum Reprod Update. 2018;24(4):455–67.
Lake JK, et al. Women’s reproductive health: the role of body mass index in early and adult life. Int J Obes Relat Metab Disord. 1997;21(6):432–8.
Lim SS, et al. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2012;18(6):618–37.
Lim SS, et al. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2019;3:CD007506.
Luke B, et al. Female obesity adversely affects assisted reproductive technology (ART) pregnancy and live birth rates. Hum Reprod. 2011;26(1):245–52.
March WA, et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544–51.
Onalan G, et al. Predictive value of glucose-insulin ratio in PCOS and profile of women who will benefit from metformin therapy: obese, lean, hyper or normoinsulinemic? Eur J Obstet Gynecol Reprod Biol. 2005;123(2):204–11.
Orvieto R, et al. Ovarian stimulation in polycystic ovary syndrome patients: the role of body mass index. Reprod Biomed Online. 2009;18(3):333–6.
Palomba S, et al. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update. 2015;21(5):575–92.
Palomba S, et al. Endometrial function in women with polycystic ovary syndrome:a comprehensive review. Hum Reprod Update. 2021;27(3):584–618.
Palomba S, et al. Oocyte competence in women with polycystic ovary syndrome. Trends Endocrinol Metab. 2017;28(3):186–98.
Pasquali R. Obesity and androgens: facts and perspectives. Fertil Steril. 2006;85(5):1319–40.
Pelanis R, et al. The prevalence of Type 2 diabetes is not increased in normal-weight women with PCOS. Hum Reprod. 2017;32(11):2279–86.
Rosenfield RL, et al. Determination of the source of androgen excess in functionally atypical polycystic ovary syndrome by a short dexamethasone androgen-suppression test and a low-dose ACTH test. Hum Reprod. 2011;26(11):3138–46.
Sam S. Obesity and polycystic ovary syndrome. Obes Manag. 2007;3(2):69–73.
Sam S, et al. Evidence for metabolic and reproductive phenotypes in mothers of women with polycystic ovary syndrome. Proc Natl Acad Sci U S A. 2006;103(18):7030–5.
Stepto NK, et al. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum Reprod. 2013;28(3):777–84.
Teede HJ, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33(9):1602–18.
Toosy S, et al. Lean polycystic ovary syndrome (PCOS): an evidence-based practical approach. J Diabetes Metab Disord. 2018;17(2):277–85.
Vaughan DA, et al. How many oocytes are optimal to achieve multiple live births with one stimulation cycle? The one-and-done approach. Fertil Steril. 2017;107(2):397-404.e393.
Welt CK, Carmina E. Clinical review: Lifecycle of polycystic ovary syndrome (PCOS): from in utero to menopause. J Clin Endocrinol Metab. 2013;98(12):4629–38.
Sundaram L, Gao H, Padigepati SR, McRae JF, Li Y, Kosmicki JA, Fritzilas N, Hakenberg J, Dutta A, Shon J, Xu J, Batzoglou S, Li X, Kai K. Predicting the clinical impact of human mutation with deep neural networks. https://doi.org/10.1038/s41588-018-0167-z.
Yildiz BO, et al. Impact of obesity on the risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(1):162–8.
Zaadstra BM, et al. Fat and female fecundity: prospective study of effect of body fat distribution on conception rates. BMJ. 1993;306(6876):484–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fouks, Y., Neuhausser, W., Ryley, D. et al. ART outcomes in lean compared to obese phenotypes of polycystic ovarian syndrome. J Assist Reprod Genet 40, 1437–1445 (2023). https://doi.org/10.1007/s10815-023-02804-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-02804-0