Abstract
The respiratory system was primarily considered the only organ affected by Coronavirus disease 2019 (COVID-19). As the pandemic continues, there is an increasing concern from the scientific community about the future effects of the virus on male and female reproductive organs, infertility, and, most significantly, its impact on the future generation. The general presumption is that if the primary clinical symptoms of COVID-19 are not controlled, we will face several challenges, including compromised infertility, infection-exposed cryopreserved germ cells or embryos, and health complications in future generations, likely connected to the COVID-19 infections of parents and ancestors. In this review article, we dedicatedly studied severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) virology, its receptors, and the effect of the virus to induce the activation of inflammasome as the main arm of the innate immune response. Among inflammasomes, nucleotide oligomerization domain-like receptor protein, pyrin domain containing 3 (NLRP3) inflammasome pathway activation is partly responsible for the inflicted damages in both COVID-19 infection and some reproductive disorders, so the main focus of the discussion is on NLRP3 inflammasome in the pathogenesis of COVID-19 infection alongside in the reproductive biology. In addition, the potential effects of the virus on male and female gonad functions were discussed, and we further explored the potential natural and pharmacological therapeutic approaches for comorbidity via NLRP3 inflammasome neutralization to develop a hypothesis for averting the long-term repercussions of COVID-19. Since activation of the NLRP3 inflammasome pathway contributes to the damage caused by COVID-19 infection and some reproductive disorders, NLRP3 inflammasome inhibitors have a great potential to be considered candidates for alleviating the pathological effects of the COVID-19 infection on the germ cells and reproductive tissues. This would impede the subsequent massive wave of infertility that may threaten the patients.
Similar content being viewed by others
Introduction
The respiratory system was primarily considered the only organ affected by Coronavirus disease 2019 (COVID-19). As the pandemic continues, there is an increasing concern from the scientific community about the future effects of the virus on male and female reproductive organs, infertility, and, most significantly, its impact on the future generation [1, 2].
While the long-term effects of COVID-19, caused by the severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2), are yet to be investigated, researchers found some insights from previously known coronaviruses [3]. However, it is still controversial whether the infection transmits sexually and impacts the next generation’s health and well-being, considering the presence of the virus in the semen and testis tissues [4]. Assessing gene alterations in patients affected by SARS-CoV-2 and understanding how this infection interrupts, various aspects of reproduction provide valuable molecular insights into the infection mechanism. This potentially improves our current interventional programs by considering infertility prevention as part of the integrated care strategies at the time of COVID-19 pandemic. In addition, reproductive societies decided to resume assisted reproductive procedures based on inaccurate findings about the long-term consequences of the virus infection [5]. The general presumption is that if the primary clinical symptoms of COVID-19 are not controlled, we will face several challenges, including compromised infertility, infection-exposed cryopreserved germ cells or embryos, and health complications in future generations, likely connected to the COVID-19 infections of parents and ancestors [6].
Since infertility is a time-sensitive situation, by discovering and controlling the main effects of COVID-19, especially at reproductive age or before puberty, the potential side effects of the disease can be suppressed in the acute phase, and subsequently, its accompanying risks on male or female reproductive organs be reduced [7].
In this review article, we dedicatedly studied SARS-CoV-2 virology, its receptors, and the effect of the virus to induce the activation of innate immune response. The activation of innate immunity in SARS-CoV-2 infection results in the systemic elevation of inflammatory biomarkers and the subsequent “cytokine release syndrome” or “cytokine storm,” which is characterized by high levels of cytokines in the plasma. Inflammasomes, as the main arm of innate immunity, play a key role in these processes. Among inflammasomes, nucleotide oligomerization domain-like receptor protein, pyrin domain containing 3 (NLRP3) inflammasome pathway activation is partly responsible for the inflicted damages in both COVID-19 infection and some reproductive disorders, so the main focus of the discussion is on NLRP3 inflammasome in the pathogenesis of COVID-19 infection alongside in the reproductive biology. We also discussed the potential effects of the virus on male and female gonad functions, and further explored the potential natural and pharmacological therapeutic approaches for comorbidity via NLRP3 inflammasome neutralization to develop a hypothesis for averting the long-term repercussions of COVID-19.
SARS-CoV-2, its receptor, and the mechanism of virus entry into cells
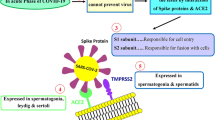
Viruses generally attach to the relevant receptors on the target cells in order to enter into the cells and infect them. So, the infectivity and disease severity of the viruses correlates with the abundance of these receptors [8]. SARS-CoV-2 is no exception. It attaches to a cellular transmembrane protein called angiotensin-converting enzyme 2 (ACE2), the key receptor for the SARS-CoV-2 cell entry, and its infectivity [9, 10]. ACE2 is also the cell surface receptor for SARS-CoV, another member of the human coronaviruses that caused the 2002–2004 outbreak of the severe acute respiratory syndrome (SARS), with about 79% sequence similarity to SARS-CoV-2 [11, 12]. A comprehensive studies have identified 380 amino acid variations between these coronaviruses, which may have resulted in functional and pathogenic divergence of new one [13] that briefly includes important mutations in the receptor-binding domains, 27 amino acid substitutions in the spike protein with a length of 1273 amino acids, and four substitutions in the C-terminal of the receptor-binding subunit S1 domain of SARS-CoV-2, compared with SARS-CoV [14, 15]. Furthermore, it was shown that SARS-CoV-2 ACE2 protein has a stronger binding affinity than SARS-CoV receptor [16, 17].
ACE2 expresses abundantly in lung alveolar epithelium and small intestine enterocytes [18], which might be the reason for the hypervulnerability of respiratory and gastrointestinal organs to COVID-19 infection [19,20,21,22]. ACE2 also expresses in the kidney, liver, spleen, and testes and could be responsible for various clinical manifestations of the disease, such as liver diseases [18, 23,24,25].
The spike (S) glycoprotein of the SARS-CoV-2 contributes significantly to the attachment of the SARS-CoV-2 to ACE2 and the fusion of the virus with the membrane of the target cell [8, 10, 26]. The spike glycoprotein also needs to be proteolytically activated by the host cells to enable the virus to enter the cell. It seems that SARS-CoV-2 infiltration of lung cells can lead to the down-regulation of membranous ACE2 in the host cell [27]. Underexpression of ACE2 has been reported in the lung tissue of SARS-CoV-infected mice [28, 29]. Angiotensin II, an octapeptide that enhances blood pressure through a vascular contraction with pro-inflammatory activities, also increases in COVID-19 patients’ circulation [27, 30]. Angiotensin II is converted from angiotensin I by ACE. In this process, ACE2 plays a protective role by converting angiotensin II to heptapeptide angiotensin-(1-7), an antagonist for angiotensin II [8, 28]. So, severe complications in SARS-CoV-2 infected patients could be explained, in part, by the loss of ACE2-expressing lung epithelium, leading to perturbation in the renin-angiotensin system that eventually escalates inflammation [31].
SARS-CoV-2 S protein is a trimer glycoprotein comprised of S1 and S2 subunits. S1 heads are the receptor-binding domains (RBD) responsible for the attachment of the virus to the ACE2 on the target cell, and S2 stalks are the responsible section for the membrane fusion. SARS-CoV-2 also uses the trans-membrane protease serine 2 (TMPRSS2) for S protein priming [32]. In fact, the S-protein of SARS-CoV-2 utilizes TMPRSS2 and ACE2 to get primed and enter into target cells [32,33,34].
The significance of the NLRP3 inflammasome pathways in the pathophysiology of SARS-CoV-2 infection
In SARS-CoV-2 infection, the activation of innate immunity leads to the systemic elevation of inflammatory biomarkers (such as D-dimer, ferritin, and C-reactive protein) and the subsequent “cytokine release syndrome” or “cytokine storm" characterized by high levels of cytokines including IL-6, TNF-α, IL-8, IL-10, IL-1RA, and CXCL10 in the plasma [35]. Inflammasomes, as an important arm of innate immunity, have a crucial role in these processes [36, 37]. NLRP3 inflammasome is one of the most studied mechanisms in innate immune cells, such as macrophages, neutrophils, dendritic cells [38,39,40], and tissues [41, 42]. NLRP3 consists of a sensor (NLRP3), an adaptor protein (apoptosis-associated speck-like protein containing a caspase recruitment domain, ASC), and an effector (procaspase 1) [43].
SARS-CoV-2 induces NLRP3 inflammasome activation by different mechanisms. It has shown that open reading frame 3a (ORF3a), a viroporin encoded by SARS-CoV-2, mediates cellular K+ efflux, a common trigger of NLRP3 activation [44]. Viroporins are virus-encoded proteins that integrate into the plasma membrane and mediate ions influx/efflux in the cell [43, 45]. Protein E, another viroporin of SARS-CoV-2, also enhances Ca2+ influx across the plasma membrane and endoplasmic reticulum membrane. Excessive intracellular calcium causes lysosomal rupture and leakage of digestive proteases into the cytosol leading to cell death [46, 47]. Mitochondrial damage and generation of reactive oxygen species (ROS) are other downstream of intracellular ion imbalance that contribute to NLRP3 inflammasome activation [47, 48]. NLRP3 inflammasome activation is a two-phase process and can be described as follows: The first step is priming, resulting in NF-kB activation and higher expression of NLRP3 inflammasome components. The second step is inflammasome assembly, during which ASC interacts with the NLRP3 and recruits procaspase-1 [49, 50]. Then, NLRP3 inflammasome activation induces cleavage of inactive procaspase-1 to active cysteine protease caspase-1, leading to activation of pro-interleukin 1β (pro-IL1β) and pro-interleukin-18 (pro-IL18) to form IL1β and IL18, respectively, both of which recruit neutrophils to the inflammatory sites and cause resultant tissue damage (Fig. 1) [49, 51]. In addition, following inflammasome activation, activated Caspase 1 cleaves gasdermin D (GSDMD), which is incorporated into the plasma membrane and forms a pore. GSDMD increases the release of IL-1β and IL-18 and causes H2O influx. It leads to cell swelling and programmed cell death “pyroptosis,” and the release of pro-inflammatory intracellular components and cytokines (Fig. 1a) [36, 52, 53].
NLRP3 Inflammasome activation, its inhibitors, and consequences of SARS-CoV-2 on reproductive organs. a NLRP3 inflammasome activation by virus-intrinsic pathway and different inhibitors. b Some of the known consequences of SARS-CoV-2 on male and female reproductive organs. Some icons were created with BioRender.com
NLRP3 inflammasome can be antagonized via several pathways in order to hinder the excessive inflammatory response. For example, the generation of destabilized subunits via proteolytic cleavage of caspase-1 leads to the termination of caspase-1 activation [54]. Chaperone-mediated suppression of NLRP3 inflammasome was also reported previously [55]. Furthermore, pyrin-only protein (POP2) and CARD-only proteins (COPs) have been shown to inhibit inflammasome assembly by interacting with ASC and caspase-1, respectively [37, 56]. Although precise cellular mechanisms regulate and terminate NLRP3 inflammasome activity, the aberration in NLRP3 inflammasome function is the main reason for tissue damage and cytokine storm in COVID-19 patients [35].
Considering the critical role of NLRP3 and inflammasomes in COVID-19 pathophysiology, better understanding of the mechanisms of antagonizing this pathway can offer new interventional strategies for current and/or future SARS-CoV outbreaks [35, 57, 58].
Potential effects of COVID-19 on the reproductive systems
Male reproductive organ: a plausible target for the SARS-CoV-2 virus
A growing body of research suggests COVID-19 can infect several organs expressing ACE2 receptors, including the male reproductive system [59, 60]. The presence of the virus or its particles in COVID-19 patient semen has been reported, leading to the speculation that the SARS-CoV-2 could penetrate the blood-testis barrier (BTB), entering the testicular micro-environment [61, 62]. Besides, scrotal discomfort and orchitis have been reported in 19% of COVID-19 men as clinical manifestations of the disease [63]. The pathophysiology mechanisms underlying these symptoms may be related to the vital role played by the ACE receptor and the cytokine storm in the manifestation and transmission of pain in COVID-19 patients [64]. Increased thickening of the seminiferous tubule basement membranes, leukocyte infiltration, orchitis, and impaired spermatogenesis have been reported in postmortem studies of SARS-CoV infected cases, and the high similarity of genome sequences between SARS-CoV and SARS-CoV-2 suggests that the same complications might develop in COVID-19 patients [2, 61, 65, 66].
SARS-CoV-2 could indirectly or directly attack the male reproductive system. Higher levels of both ACE2 and TMPRSS2 were reported in seminiferous tubules of COVID-19 patients [67], and the elevated concentration of ACE2 receptors in this tissue was reported to lead to lower spermatogenesis [60]. ACE2 receptor could mediate activation of NLRP3 inflammasome in male reproductive organs in two ways: first, facilitating SARS-CoV-2 entry into the cell and triggering NLRP3 activation in the testicular tissue, particularly in Leydig and Sertoli cells, disrupting the organ functions [68]. Furthermore, COVID-19-induced ACE2 downregulation in testicular cells promotes Angiotensin II upregulation, which in turn activates the NLRP3 inflammasome and pro-inflammatory response in testes [69, 70].
On the contrary, several studies reported the absence of virus (or viral particles) in patients’ semen and summarized that COVID-19 directly damage testes by increasing pro-inflammatory cytokines in the organ [71, 72]. A recent study on the testes of 12 deceased COVID-19 patients demonstrated that although RT-PCR did not detect the virus genome in the semen of 90% of patients, and the electron microscopy yielded negative results in all cases, Sertoli and Leydig cells were extensively damaged in infected patients [73]. Injured Sertoli cells lose their ability to nourish developing sperm cells, eventually disrupting spermatogenesis [74]. The damage to Leydig cells also dysregulates testosterone production [75], which could explain the decreased ratio of testosterone to LH and FSH to LH in COVID-19 male patients, as such disruption pattern is highly associated with Leydig cells dysfunction and hypogonadism [76,77,78].
In addition, TMPRSS2 expression has been reported in spermatogonial stem cells, epididymal luminal epithelial cells, elongated spermatids, and prostate luminal epithelial cells. As a result, these tissues can potentially serve as a host if they are exposed to the virus [79].
Female reproductive system is vulnerable to SARS-CoV-2 infection
Until now, there is known and unknown information about SARS-CoV-2 in female reproductive organs [80]. Meta-analysis of high-throughput gene and protein expression data from online repositories revealed that various reproductive organs and tissues are possible SARS-CoV-2 contamination sites [1, 81]. Several cell types in human female reproductive organs, such as fallopian tubes, the ovary, cervix, and endometrium, have been shown to express putative receptors (like ACE2) for SARS-CoV-2 infection [2, 82]. ACE2 plays crucial roles in the different functions of the ovary, including promoting steroid hormone production and secretion, aiding follicle growth and oocyte development, regulating early stages of ovulation, and maintaining the function of the corpus luteum [83,84,85].
Recent studies revealed the effects of SARS-CoV-2 infection on the different aspects of female fertility and investigated whether the female reproductive system is vulnerable to SARS-CoV-2 infection [86,87,88,89]. Expression patterns of SARS-CoV-2 receptor proteins, ACE2 and TMPRSS2, in endometrial tissue, uterine smooth muscle, decidual stromal cells, and extravillous trophoblast suggest the vulnerability of these organs to viral infection [90]. Interestingly, the susceptibility to diseases rises with the patient age because ACE2 shows a moderately increased endometrial expression with age [91]. Moreover, altered ovarian reserve, considerable menstrual changes, and hormonal imbalance were reported in females with SARS-CoV-2 infection [86]. Bentov et al. (2021) evaluated three groups of females in a cohort study, including vaccinated, recovered from confirmed COVID-19, and non-vaccinated, and reported that COVID-19 has no negative impact on ovarian follicle activities such as the number of retrieved oocytes, oocyte yield, mature oocytes, and oocyte quality biomarkers in the follicular fluid [92]. Interestingly, a comparison of FSH levels in patients undergoing IVF treatment before and after the pandemic revealed a trend of increased FSH levels in post-pandemic patients, which has been associated with lower pregnancy rates [93]. However, anti-Müllerian hormone (AMH) levels were not different between women with COVID-19 and the control group in any of the published studies [86, 94, 95]. Wang et al., in a retrospective cohort study, compared SARS-COV-2 exposed and unexposed women and reported a significant difference in blastocyst formation rate but not in the number of recovered oocytes, maturation rate, fertilization rate, and biochemical and clinical pregnancies [96]. On the other hand, Herrero et al. [88] and Orvieto et al. [97] reported a lower number of retrieved and mature oocytes and a reduced number of top-quality embryos in the women exposed to SARS-COV-2.
Taken together, the expression patterns of the ACE2 and TMPRSS2 in the male and female reproductive tissues support the idea that the virus can infiltrate these compartments and promote a chain of pathophysiological processes leading to tissue architecture change and host cell apoptosis. Some of the known consequences of SARS-CoV-2 on male and female reproductive organs are shown in Fig 1b.
SARS-CoV-2, fertility disorders, and their common players
Since SARS-CoV-2 infection causes a variety of humoral and cellular immunological responses, one way to overcome COVID-19 long-term implications and reverse the deterioration of patient health is to control cytokine storm or inflammation responses [98]. In this context, several candidates have been suggested. Among them, NLRP3 is a common immune mediator in organ damage, either respiratory system or reproductive tissues, accompanied by multisystem infections [47]. Excess activation of NLRP3, as the most important and best-studied inflammasome, and its signaling pathway in male and female reproductive systems have recently been shown to induce an imbalance of inflammatory and pro-inflammatory cytokines, resulting in infertility, miscarriage, preeclampsia, gestational diabetes, preterm labor, reproductive aging, PCOS, and endometriosis [99, 100]. Therefore, developing an approach to its blockade might be a hopeful and promising treatment strategy for both comorbidities [101].
Inflammasome in reproductive biology
NLRP3 in male infertility
Several inflammatory and non-inflammatory mechanisms have been associated with male sub/infertility pathologies. Some reports are showing the involvement of NLRP3 inflammasome in conditions leading to male infertility, including spinal cord injury (SCI), varicocele, and, more recently, COVID-19. In testes, NLRP3 is expressed in Sertoli cells and its overexpression by infection or sterile stimuli can lead to dysregulation of spermatogenesis and male infertility [102]. Studies revealed that following SCI, BTB disruption, leukocyte infiltration, increased apoptosis of sperm cells, and elevated inflammatory cytokines lead to induction of an inflammatory niche in the testis [103,104,105]. NLRP3 inflammasome and its end products (IL1β and IL-18) are elevated in the semen of these patients, which has a toxic effect on sperm quality. They present lower motility and viability while the normal concentration of sperm compared to the control group. Ibrahim et al. supported these findings in their study of treating semen with an antibody against ASC, leading to the inhibition of NLRP3 inflammasome and a subsequent significant improvement in sperm motility in SCI patients [106]. A research study found that malondialdehyde, which serves as a marker of oxidative stress, increased significantly in the testis of rats after seven days of SCI. The simultaneous increase in malondialdehyde and NLRP3 expression suggests that the generation of high levels of ROS in the testis might be responsible for NLRP3 inflammasome activation and impaired function of the testis in these patients [107].
Studying infertile men with oligozoospermia and accessory gland inflammatory alterations, Milardi et al. reported that the treatment with prednisolone, which acts as an anti-inflammatory agent, could improve sperm motility and concentration, possibly by reducing leukocyte infiltration and pro-inflammatory cytokines in their genital tract [108]. Considering the role of leukocytes in inflammation response, in some experiments, the leukocytes were removed from the sperm suspension to evaluate the contribution of sperm alone to NLRP3 inflammasome activation. The findings showed that the high expression of ASC and caspase-1 in these patients is not a sequel of either leukocytes or sperm cells, but both act in concert. The generation of high amounts of reactive oxygen species (ROS) by leukocytes and sperm cells results in oxidative stress, which is a significant reason of inflammasome activation [109,110,111].
Varicocele, a major cause of male infertility, has been shown to be associated with NLRP3 inflammasome activation [112]. A recent study confirmed that the level of IL-1b, ASC, and NLRP3 significantly increased in the seminal plasma of varicocele patients and that resveratrol, a powerful antioxidant, diminished the expression levels of NLRP3 inflammasome components in the testis of varicocele rats. Therefore, ROS production has been linked to NLRP3 inflammasome activation in the testis of infertile varicocele men [113, 114].
Muckle-Wells syndrome, a rare auto-inflammatory disorder caused by gain-of-function mutations in NLRP3, may contribute to perturbed spermatogenesis and male sterility [115]. Overproduction of IL-1b due to the permanent activity of the NLRP3 inflammasome is demonstrated to inhibit testosterone production in Leydig cells, which is considered a key contributing factor of male infertility in this syndrome [102].
In testicular ischemia/reperfusion injury, inflammatory cell infiltration, high levels of ROS, and NLRP3 inflammasome activation are particularly involved in testicular dysfunction and disturbed spermatogenesis [116, 117]. A study on a mouse model of testicular ischemia/reperfusion injury reported NLRP3 signaling blockade resulted in reduced pro-inflammatory cytokines and improved testicular function [118].
Obesity linked to chronic inflammation and inflammatory status in reproductive tissue might lead to male infertility. A study reported that high-fat-diet-induced obese mouse model shows poor sperm quality and a lower fertility rate. In a subsequent study on a high-fat-diet-induced obese mouse model and overweight males, they showed a direct relation between obesity-related infertility and high levels of NLRP3 and pro-inflammatory cytokines in the epididymis, prostate, and especially in the testis, suggesting an association between the NLRP3 inflammasome complex and obesity-induced infertility [119, 120].
Inflammasome and female infertility
The NLRP3 inflammasome has been linked to several different high-risk female reproductive diseases, including recurrent spontaneous abortion (RSA), polycystic ovarian syndrome (PCOS), especially in overweight patients, endometriosis, gestational diabetes mellitus, and preterm birth [121].
The pathophysiology of PCOS is still controversial, and numerous studies have suggested that chronic inflammation is implicated. Some PCOS women appear to have higher levels of androgen, oxidative stress, and free fatty acid (FFA) elements that act as danger signals to trigger the inflammasome pathway, particularly the NLRP3 one [122]. The cytokines implicated in the NLRP3 pathway have critical roles in regulating ovarian steroidogenesis and ovarian follicle development. Also, IL-18 expression was dramatically enhanced in PCOS patients. On the other hand, because IL-1β reduces adipose tissue insulin sensitivity by decreasing insulin signaling, reducing its activity or synthesis may improve insulin signaling [123, 124].
Aging is a physiological process in which various parameters play roles, entailing progressive destruction of physiological and metabolic homeostasis, inflammation included [125, 126]. Female infertility is a process that can be affected by aging as one of its physiological contributors [127, 128]. Studies have shown that NLRP3 is involved in the process of infertility related to physiological aging. Interestingly, the ovarian aging process can be hindered, to some extent, by using NLRP3 inhibitors [125, 126].
As mentioned earlier, inflammation is involved in different aspects of a normal and pathological pregnancy, such as implantation, pregnancy maintenance, and parturition [129,130,131]. More interestingly, various components of the inflammasome pathway are detectible in gestational tissue. NLRP3 has been detected in peripheral leukocytes of pregnant women, in placentae throughout the pregnancy, in the chorioamniotic membranes, as well as in myometrial tissues at term pregnancy [132,133,134,135]. In contrast, an unrestricted level of inflammation can have negative impacts on female fertility [136]. Intra-amniotic infection and sterile intra-amniotic inflammation are two conditions associated with inflammation that may result in pathological at-term pregnancies. The former is inflammation with detectible microorganisms in the amniotic fluid, but the latter is inflammation without detectible microorganisms [137]. Studies have demonstrated that NLRP3, activated forms of caspase-1, IL-1B, and IL-18, are involved in the inflammation that occurs in histologic chorioamnionitis [138,139,140]. Therefore, Gomez-Lopez et al. suggested that inflammasomes play roles in the pathological inflammation that occurs in the pregnancies at term, which are associated with intra-amniotic infections [141]. It has also been revealed that inflammasome and NLRP3 are involved in the preterm labor associated with sterile intra-amniotic inflammation [142, 143]. NLRP3 inflammasome plays a role in the progression of preeclampsia, as the escalated levels of NLRP3, caspase-1, and IL-1B in the placentas of women with severe preeclampsia has been reported [144, 145].
In summary, based on the above-mentioned studies, inflammation, which comes from the earlier activation of the inflammasome, is a game-changing factor in physiological and pathological pregnancy, female reproductive disorders; on the other side, uncontrolled activation of inflammasome can also affect female fertility.
NLRP3 inflammasome inhibitors: killing two birds with one stone
Since NLRP3 inflammasome is linked to a wide variety of disorders, there is a substantial amount of investment in the scientific community to find inhibitors of NLRP3 inflammasome that are both efficient and reliable. An array of targets is available for suppressing the NLRP3 inflammasome by utilizing its complicated signaling cascade [146]. For instance, inhibition of NLRP3 inflammasome activation, suppression of upstream and/or downstream signals, blockade of inflammasome assembly and Gasdermin D (GSDMD) cleavage, inhibition of caspase-1 activation, and neutralization of the cytokines produced by the NLRP3 inflammasome can all be tailored for the prospective inhibition of the NLRP3 inflammasome [147].
Because SARS-CoV-2 can provoke humoral and cellular immunological responses, one proposed approach to overcome COVID-19 long-term implications is to reduce inflammasome activation using inhibitors targeting the NLRP3 inflammasome and its downstream pathways [57]. Taken together, pathological effects on reproductive tissues and germ cells may also be prevented by using inflammasome inhibitor drugs.
Natural NLRP3 inflammasome inhibitors
Dietary micronutrients may have preventive or therapeutic effects on NLRP3 as the upstream activators of inflammatory responses [148]. The immune system-boosting, antiviral, and anti-inflammatory properties of different nutraceuticals have been well-documented [149]. Among them, 1,25-dihydroxyvitamin D3 (1,25(OH)2 D3, VitD3, calcitriol), γ-tocopherol (vitamin E), vitamin C (ascorbic acid), and cinnamaldehyde (organic compound in cinnamon) could be effective against inflammation via down-regulation of the inflammatory response mediated by NF-kB/NLRP3 [150,151,152,153]. Interestingly, serum vitamin D levels are shown to be inversely associated with the prevalence or severity of COVID-19 in several studies [154, 155].
Nicotinic acid (NA, vitamin B3) and pyridoxine (vitamin B6) exert their anti-inflammatory effects by inhibiting NLRP3-dependent caspase-1 processing and the subsequent secretion of mature IL-1β and IL-18 [156, 157]. VitD3 (as the co-activator of vitamin D receptor (VDR)) also suppresses NLRP3 inflammasome via a different mechanism, including inhibiting caspase-1 activation, reducing IL-1β secretion, and preventing NLRP3-mediated ASC oligomerization. Interestingly, there is a direct interaction between VDR and NLRP3 by which vitamin D treatment results in attenuating the inflammatory response mediated by NF-kB/NLRP3 [150].
Riboflavin (vitamin B2) prevents mitochondrial ROS production and DNA damage, which triggers the NLRP3 inflammasome assembly. Furthermore, this vitamin disrupts the activity of caspase-1 and inhibits the absence in melanoma 2 (AIM2), NLR family-CARD domain containing 4 (NLRC4), and non-canonical inflammasomes [158]. It seems that zinc has a double-edged sword role in the inflammatory pathways [159, 160]. Both the deficiency and high doses have adverse effects. In deficient samples, zinc promotes autophagy, activates the protein expression of ubiquitin, and suppresses high levels of NLRP3 [161]. Zinc deficiency has been reported to be associated with NLRP3 inflammasome activation and the production of IL-1β in macrophages by nuclear factor erythroid 2-related factor 2 (Nrf2) pathway activation [162].
Pharmacological NLRP3 inflammasome inhibitors
Until now, various inhibitors for each component of the inflammasome signaling cascade acting directly or indirectly have been presented through in vitro and in vivo experimental studies. Some of them directly inhibit NLRP3, such as the MCC950 (specifically binds to NLRP3), 3,4-methylenedioxy-β-nitrostyrene (MNS), CY-09, and Tranilast (N-[3′ ,4′ -dimethoxycinnamoyl]-anthranilic acid, TR) [163]. Other inhibitors, in contrast, target NLRP3 indirectly; for instance, glyburide inhibits ATP-sensitive K+ channels and ASC aggregation [147, 164, 165].
Some inhibitors can act on different targets simultaneously, such as parthenolide, which is an inhibitor with a broad spectrum and various anti-inflammatory characteristics. NF-kB, caspase-1, IKKβ, and several inflammasomes, including NLRC4, NLRP1, and NLRP3, are among its targets [166]. The powerful and irreversible caspase-1 inhibitor known as ac-YVAD-cmk has been shown to have anti-apoptotic, antipyroptotic, and anti-inflammatory properties [167]. Disulfiram (an FDA-approved drug) inhibits the GSDMD pores formation and hence suppresses the IL-1β release and subsequent pyroptosis of cells [163].
Interestingly, there are NLRP3 inhibitors with multiple functions that improve some infertility pathology. Pioglitazone, an insulin sensitizer, is used to promote ovulation in PCOS patients with insulin resistance; on the other hand, it is a potent NLRP3 inhibitor and exerts an anti-inflammatory effect, declining NLRP3 levels and downstream inflammatory cytokines in patients with diabetes mellitus [168, 169]. MCC950 and CY-09 were used to attenuate dysmenorrhea and reduce the development of endometriotic lesions by suppressing the NLRP3, respectively [170, 171]. In addition, Tranilast improves the semen parameters in severe oligoasthenozoospermia [172].
Conclusion
Female and male reproductive organs express ACE2 and TMPRSS2 receptors; therefore, these systems are vulnerable to SARS-CoV-2 infection. NLRP3 inflammasome and its end pathway products (IL1β and IL-18) activation are responsible for the inflicted damages in both COVID-19 infection and some reproductive pathophysiology. An array of NLRP3 inflammasome inhibitors that are both efficient and reliable is available for suppressing the NLRP3 inflammasome by utilizing many targets in its complicated signaling cascade.
Expert opinion
Since COVID-19 infection and some reproductive disorders have common players for the inflicted damages especially NLRP3 inflammasome pathway activation, its inhibitors have a great potential to be considered candidates for alleviating the long-term repercussions of the COVID-19 infection on the germ cells or embryos, reproductive tissues, and health complications in future generations. This would impede the subsequent massive wave of infertility that may threaten the patients. Therefore, better understanding of the mechanisms of antagonizing these pathways can offer new interventional strategies for current and/or future SARS-CoV outbreaks.
References
Sun J. The hypothesis that SARS-CoV-2 affects male reproductive ability by regulating autophagy. Med Hypotheses. 2020;143:110083.
Zupin L, Pascolo L, Zito G, et al. SARS-CoV-2 and the next generations: which impact on reproductive tissues? J Assist Reprod Genet. 2020;37(10):2399–403.
Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19(3):141–54 2021/03/01.
Sengupta P, Leisegang K, Agarwal A. The impact of COVID-19 on the male reproductive tract and fertility: a systematic review. Arab J Urol. 2021;19(3):423–36.
Huri M, Noferi V, Renda I, et al. The COVID-19 pandemic impact on the outcome of medically assisted reproduction pregnancies. Front reprod health. 24.
Blumenfeld Z. Possible impact of COVID-19 on fertility and assisted reproductive technologies. Fertil Steril. 2020;114(1):56–7.
Veiga A, Gianaroli L, Ory S, et al. Assisted reproduction and COVID-19: a joint statement of ASRM, ESHRE and IFFS∗. Fertil Steril. 2020;114(3):484–5.
Matheson NJ, Lehner PJ. How does SARS-CoV-2 cause COVID-19? Science. 2020;369(6503):510–1.
Andersen KG, Rambaut A, Lipkin WI, et al. The proximal origin of SARS-CoV-2. Nat Med. 2020;26(4):450–2.
Huang Q, Herrmann A. Fast assessment of human receptor-binding capability of 2019 novel coronavirus (2019-nCoV). BioRxiv. 2020.
Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–4.
Organization WH. Consensus document on the epidemiology of severe acute respiratory syndrome (SARS). 2003. World Health Organization; 2005.
Wu A, Peng Y, Huang B, et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27(3):325–8.
Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The lancet. 2020;395(10224):565–74.
Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–3.
Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–3.
Shang J, Ye G, Shi K, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–4.
Hamming I, Timens W, Bulthuis M, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–7.
Soltani S, Zakeri A, Rezayat SA, et al. A systematic literature review on COVID-19, clinical manifestation, laboratory and radiologic features. Advances in Human Biology. 2021;11(1):26.
Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9.
Gu J, Han B, Wang J. COVID-19: gastrointestinal manifestations and potential fecal–oral transmission. Gastroenterology. 2020;158(6):1518–9.
Banerjee S, Gupta J, Kanaujia A. COVID 19 pandemic, mechanism of pathogenesis, preventions and possible cures to save humanity: a study. J Reprod Infertil. 2020;8(2):18–21.
Hosseini P, Afzali S, Karimi M, et al. The coronavirus disease 2019 and effect on liver function: a hidden and vital interaction beyond the respiratory system. Reviews in Medical Microbiology. 2021;33:e161–79.
Reis AB, Araújo FC, Pereira VM, et al. Angiotensin (1–7) and its receptor Mas are expressed in the human testis: implications for male infertility. J Mol Histol. 2010;41(1):75–80.
Verma S, Saksena S, Sadri-Ardekani H. ACE2 receptor expression in testes: implications in coronavirus disease 2019 pathogenesis. Biol Reprod. 2020;103(3):449–51.
Li F. Receptor recognition mechanisms of coronaviruses: a decade of structural studies. J Virol. 2015;89(4):1954–64.
Banu N, Panikar SS, Leal LR, et al. Protective role of ACE2 and its downregulation in SARS-CoV-2 infection leading to macrophage activation syndrome: Therapeutic implications. Life Sci. 2020;256:117905.
Kuba K, Imai Y, Rao S, et al. Lessons from SARS: control of acute lung failure by the SARS receptor ACE2. J Mol Med. 2006;84(10):814–20.
Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med. 2005;11(8):875–9 2005/08/01.
Satou R, Penrose H, Navar LG. Inflammation as a regulator of the renin-angiotensin system and blood pressure. Curr Hypertens Rep. 2018;20(12):1–9.
Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020;35(3):266–71.
Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271-280.e8.
Shang J, Wan Y, Luo C, et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci. 2020;117(21):11727–34.
Hoffmann M, Kleine-Weber H, Krüger N, et al. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. BioRxiv. 2020.
Freeman TL, Swartz TH. Targeting the NLRP3 inflammasome in severe COVID-19. Front Immunol. 2020;11:1518.
Yap JK, Moriyama M, Iwasaki A. Inflammasomes and pyroptosis as therapeutic targets for COVID-19. J Immunol. 2020;205(2):307–12.
van den Berg DF, Te Velde AA. Severe COVID-19: NLRP3 inflammasome dysregulated. Front Immunol. 2020;11:1580.
Martinon F, Pétrilli V, Mayor A, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–41.
Goldberg EL, Asher JL, Molony RD, et al. β-Hydroxybutyrate deactivates neutrophil NLRP3 inflammasome to relieve gout flares. Cell Rep. 2017;18(9):2077–87.
Kool M, Pétrilli V, De Smedt T, et al. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immun J. 2008;181(6):3755–9.
Kummer JA, Broekhuizen R, Everett H, et al. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem. 2007;55(5):443–52.
Freeman L, Guo H, David CN, et al. NLR members NLRC4 and NLRP3 mediate sterile inflammasome activation in microglia and astrocytes. J Exp Med. 2017;214(5):1351–70.
Shah A. Novel coronavirus-induced NLRP3 inflammasome activation: a potential drug target in the treatment of COVID-19. Front Immunol. 2020;11:1021.
Chen I-Y, Moriyama M, Chang M-F, et al. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front Microbiol. 2019;10:50.
Nieva JL, Madan V, Carrasco L. Viroporins: structure and biological functions. Nat Rev Microbiol. 2012;10(8):563–74.
Inal J. COVID-19 comorbidities, associated procoagulant extracellular vesicles and venous thromboembolisms: a possible link with ethnicity? Br J Haematol. 2020;190(4):e218–20.
de Rivero Vaccari JC, Dietrich WD, Keane RW, et al. The inflammasome in times of COVID-19. Front Immunol. 2020;11:2474.
Farag N, Breitinger U, Breitinger H, et al. Viroporins and inflammasomes: a key to understand virus-induced inflammation. Int J Biochem Cell Biol. 2020;122:105738.
Khan RN, Hay DP. A clear and present danger: inflammasomes DAMPing down disorders of pregnancy. Hum Reprod Update. 2015;21(3):388–405.
Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–32.
Huang Q, Wu X, Zheng X, et al. Targeting inflammation and cytokine storm in COVID-19. Pharmacol Res. 2020;159:105051.
Guo H, Ting JPY. Inflammasome assays in vitro and in mouse models. Curr Protoc Immunol. 2020;131(1):e107.
Xia S, Zhang Z, Magupalli VG, et al. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature. 2021;593:607–11.
Boucher D, Monteleone M, Coll RC, et al. Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. J Exp Med. 2018;215(3):827–40.
Mayor A, Martinon F, De Smedt T, et al. A crucial function of SGT1 and HSP90 in inflammasome activity links mammalian and plant innate immune responses. Nat Immunol. 2007;8(5):497–503.
Ratsimandresy RA, Chu LH, Khare S, et al. The PYRIN domain-only protein POP2 inhibits inflammasome priming and activation. Nat Commun. 2017;8(1):1–15.
Zhao N, Di B, Xu L-l. The NLRP3 inflammasome and COVID-19: Activation, pathogenesis and therapeutic strategies. Cytokine Growth Factor Rev. 2021;61:2–15.
Pan P, Shen M, Yu Z, et al. SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation. Nat Commun. 2021;12(1):1–17.
He Y, Wang J, Ren J, et al. Effect of COVID-19 on male reproductive system–a systematic review. Front Endocrinol. 2021;12:677701.
Wang Z, Xu X. scRNA-seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS-CoV-2 infection in spermatogonia, Leydig and Sertoli cells. Cells. 2020;9(4):920.
Achua JK, Chu KY, Ibrahim E, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections on testis. World J Mens Health. 2021;39(1):65.
Madjunkov M, Dviri M, Librach C. A comprehensive review of the impact of COVID-19 on human reproductive biology, assisted reproduction care and pregnancy: a Canadian perspective. J Ovarian Res. 2020;13(1):1–18.
Pan F, Xiao X, Guo J, et al. No evidence of SARS-CoV-2 in 393 semen of males recovering from COVID-19. Fertil Steril. 2020;113(6):1135–9.
Rani M, Uniyal A, Tiwari V, et al. Decrypting the cellular and molecular intricacies associated with COVID-19-induced chronic pain. Metab Brain Dis. 2022;37:2629–42 2022/07/18.
Xu J, Qi L, Chi X, et al. Orchitis: a complication of severe acute respiratory syndrome (SARS). Biol Reprod. 2006;74(2):410–6.
Malik YA. Properties of coronavirus and SARS-CoV-2. Malays J Pathol. 2020;42(1):3–11.
Ma X, Guan C, Chen R, et al. Pathological and molecular examinations of postmortem testis biopsies reveal SARS-CoV-2 infection in the testis and spermatogenesis damage in COVID-19 patients. Cell Mol Immunol. 2021;18(2):487–9.
Illiano E, Trama F, Costantini E. Could COVID-19 have an impact on male fertility? Andrologia. 2020;52(6):e13654.
Kai H, Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors—lessons from available evidence and insights into COVID-19. Hypertens Res. 2020;43(7):648–54.
Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2(7):247–57.
Peirouvi T, Aliaghaei A, Eslami Farsani B, et al. COVID-19 disrupts the blood–testis barrier through the induction of inflammatory cytokines and disruption of junctional proteins. Inflamm Res. 2021;70(10):1165–75.
Abdelhamid MHM, Fellah AA, Elmarghani A, et al. An assessment of men semen alterations in SARS-CoV-2: is fever the principal concern? Reprod Sci. 2022; 2022/02/22.
Yang M, Chen S, Huang B, et al. Pathological findings in the testes of COVID-19 patients: clinical implications. Eur Urol Focus. 2020;6(5):1124–9.
Griswold MD. The central role of Sertoli cells in spermatogenesis. In: Seminars in cell & developmental biology. Elsevier; 1998.
Zirkin BR, Papadopoulos V. Leydig cells: formation, function, and regulation. Biol Reprod. 2018;99(1):101–11.
Ma L, Xie W, Li D, et al. Effect of SARS-CoV-2 infection upon male gonadal function: a single center-based study. MedRxiv. 2020.
Ma L, Xie W, Li D, et al. Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J Med Virol. 2021;93(1):456–62.
Selvaraj K, Ravichandran S, Krishnan S, et al. Testicular atrophy and hypothalamic pathology in COVID-19: possibility of the incidence of male infertility and HPG axis abnormalities. Reprod Sci. 2021;28(10):2735–42 2021/10/01.
Song H, Seddighzadeh B, Cooperberg MR, et al. Expression of ACE2, the SARS-CoV-2 receptor, and TMPRSS2 in prostate epithelial cells. Eur Urol. 2020;78(2):296.
Bazrafkan M, Hosseini E, Nazari M, et al. NLRP3 inflammasome: a joint, potential therapeutic target in management of COVID-19 and fertility problems. J Reprod Immunol. 2021;148:103427.
Kharbach Y, Khallouk A. Male genital damage in COVID-19 patients: are available data relevant? Asian journal of urology. 2021;8(3):324–6.
Hikmet F, Méar L, Edvinsson Å, et al. The protein expression profile of ACE2 in human tissues. Mol Syst Biol. 2020;16(7):e9610.
Stefanello JR, Barreta MH, Porciuncula PM, et al. Effect of angiotensin II with follicle cells and insulin-like growth factor-I or insulin on bovine oocyte maturation and embryo development. Theriogenology. 2006;66(9):2068–76.
Ferreira R, Oliveira JF, Fernandes R, et al. The role of angiotensin II in the early stages of bovine ovulation. Reproduction. 2007;134(5):713–9.
Stanley KE, Thomas E, Leaver M, et al. Coronavirus disease-19 and fertility: viral host entry protein expression in male and female reproductive tissues. Fertil Steril. 2020;114(1):33–43.
Carp-Veliscu A, Mehedintu C, Frincu F, et al. The effects of SARS-CoV-2 infection on female fertility: a review of the literature. Int J Environ Res Public Health. 2022;19(2).
Malloy SM, Bradley DE. The relationship between perceived stress during the COVID-19 pandemic and menstrual cycles and symptoms. Fertil Steril. 2021;116(3):e72.
Herrero Y, Pascuali N, Velázquez C, et al. SARS-CoV-2 infection negatively affects ovarian function in ART patients. Biochim Biophys. 2022;1868(1):166295.
Yilmaz N, Buyuk GN, Ersak DT, et al. The impact of COVID 19 pandemic on pregnancy outcomes of ART cycles. Journal of Infertility and Reproductive Biology. 2021;9(4):155–9.
Li Q, Wang W, Pei C, et al. Expression of SARS-CoV-2 entry genes ACE2 and TMPRSS2 at single cell resolution in the peripartum decidua. Am J Transl Res. 2021;13(5):4389–400.
Henarejos-Castillo I, Sebastian-Leon P, Devesa-Peiro A, et al. SARS-CoV-2 infection risk assessment in the endometrium: viral infection-related gene expression across the menstrual cycle. Fertil Steril. 2020;114(2):223–32.
Bentov Y, Beharier O, Moav-Zafrir A, et al. Ovarian follicular function is not altered by SARS-CoV-2 infection or BNT162b2 mRNA COVID-19 vaccination. Hum Reprod. 2021;36(9):2506–13.
Martel RA, Shaw J, Blakemore JK. Trends in FSH levels and cycle completion rates in women undergoing assisted reproductive technology (ART) before and during the COVID-19 pandemic. Fertil Steril. 2021;116(1, Supplement):e33 2021/07/01/.
Li K, Chen G, Hou H, et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online. 2021;42(1):260–7.
Kolanska K, Hours A, Jonquière L, et al. Mild COVID-19 infection does not alter the ovarian reserve in women treated with ART. Reprod Biomed Online. 2021;43(6):1117–21.
Wang M, Yang Q, Ren X, et al. Investigating the impact of asymptomatic or mild SARS-CoV-2 infection on female fertility and in vitro fertilization outcomes: a retrospective cohort study. EClinicalMedicine. 2021;38:101013.
Orvieto R, Segev-Zahav A, Aizer A. Does COVID-19 infection influence patients’ performance during IVF-ET cycle?: an observational study. Gynecol Endocrinol. 2021;37(10):895–7.
Tang L, Yin Z, Hu Y, et al. Controlling cytokine storm is vital in COVID-19. Front Immunol. 2020;3158.
de Rivero Vaccari JP. The inflammasome in reproductive biology: a promising target for novel therapies. Front Endocrinol. 2020;11:8.
Walenta L, Schmid N, Schwarzer JU, et al. NLRP3 in somatic non-immune cells of rodent and primate testes. Reproduction. 2018;156(3):231–8.
Paniri A, Akhavan-Niaki H. Emerging role of IL-6 and NLRP3 inflammasome as potential therapeutic targets to combat COVID-19: role of lncRNAs in cytokine storm modulation. Life Sci. 2020;257:118114.
Walenta L. Mechanisms of sterile inflammation in the testis. lmu; 2018.
Sánchez-Ramos A, Vargas-Baquero E, Martin-de Francisco F, et al. Early spermatogenesis changes in traumatic complete spinal cord-injured adult patients. Spinal Cord. 2017;55(6):570–4.
Noh JH, Park SM, Oh BR, et al. The effect of spinal cord injury on spermatogenesis in rats. Korean J Urol. 1997;38(10):1023–32.
Dulin JN, Moore ML, Gates KW, et al. Spinal cord injury causes sustained disruption of the blood-testis barrier in the rat. PLoS ONE. 2011;6(1):e16456.
Ibrahim E, Castle S, Aballa T, et al. Neutralization of ASC improves sperm motility in men with spinal cord injury. Hum Reprod. 2014;29(11).
Bazrafkan M, Nikmehr B, Shahverdi A, et al. Lipid peroxidation and its role in the expression of NLRP1a and NLRP3 genes in testicular tissue of male rats: a model of spinal cord injury. Iran Biomed J. 2018;22(3):151.
Milardi D, Luca G, Grande G, et al. Prednisone treatment in infertile patients with oligozoospermia and accessory gland inflammatory alterations. Andrology. 2017;5(2):268–73.
Whittington K. Relative contribution of leukocytes and of spermatozoa to reactive oxygen species production in human sperm suspensions. Int J Androl. 1999;22(4):229–35.
Fraczek M, Kurpisz M. Inflammatory mediators exert toxic effects of oxidative stress on human spermatozoa. J Androl. 2007;28(2):325–33.
Cruz CM, Rinna A, Forman HJ, et al. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282(5):2871–9.
Majd NE, Sadeghi N, Tavalaee M, et al. Evaluation of oxidative stress in testis and sperm of rat following induced varicocele. Urol J. 2019;16(3):300–6.
Hajipour E, Mashayekhi FJ, Mosayebi G, et al. Resveratrol decreases apoptosis and NLRP3 complex expressions in experimental varicocele rat model. Iran J Basic Med Sci. 2018;21(2):225.
Baazm M, Ghafarizadeh AA, Kamran ARN, et al. Presence of the NLRP3 inflammasome components in semen of varicocele patients. International journal of fertility & sterility. 2020;14(1):46.
Tran T-A, Koné-Paut I, Marie I, et al. Muckle-Wells syndrome and male hypofertility: a case series. In: Seminars in arthritis and rheumatism. Elsevier; 2012.
Ozbal S, Ergur BU, Erbil G, et al. The effects of α-lipoic acid against testicular ischemia-reperfusion injury in rats. Scientific World Journal. 2012;2012.
Minutoli L, Puzzolo D, Rinaldi M, et al. ROS-mediated NLRP3 inflammasome activation in brain, heart, kidney, and testis ischemia/reperfusion injury. Oxid Med Cell Longev. 2016;2016.
Minutoli L, Antonuccio P, Irrera N, et al. NLRP3 inflammasome involvement in the organ damage and impaired spermatogenesis induced by testicular ischemia and reperfusion in mice. J Pharmacol Exp Ther. 2015;355(3):370–80.
Pini T, Parks J, Russ J, et al. Obesity significantly alters the human sperm proteome, with potential implications for fertility. J Assist Reprod Genet. 2020;37(4):777–87.
Fan W, Xu Y, Liu Y, et al. Obesity or overweight, a chronic inflammatory status in male reproductive system, leads to mice and human subfertility. Front Physiol. 2018;8:1117.
Zhou F, Li C, Zhang S-Y. NLRP3 inflammasome: a new therapeutic target for high-risk reproductive disorders? Chin Med J (Engl). 2021;134(01):20–7.
Rostamtabar M, Esmaeilzadeh S, Karkhah A, et al. Elevated expression of IL-18 but not IL-1β gene is associated with NALP3 and AIM2 inflammasome in Polycystic Ovary Syndrome. Gene. 2020;731:144352.
Salmassi A, Fattahi A, Nouri M, et al. Expression of mRNA and protein of IL-18 and its receptor in human follicular granulosa cells. J Endocrinol Invest. 2017;40(4):447–54.
Gao D, Madi M, Ding C, et al. Interleukin-1β mediates macrophage-induced impairment of insulin signaling in human primary adipocytes. Am J Physiol Endocrinol. 2014;307(3):E289–304.
Cordero MD, Williams MR, Ryffel B. AMP-activated protein kinase regulation of the NLRP3 inflammasome during aging. Trends Endocrinol Metab. 2018;29(1):8–17.
Navarro-Pando JM, Alcocer-Gómez E, Castejón-Vega B, et al. Inhibition of the NLRP3 inflammasome prevents ovarian aging. Sci Adv. 2021;7(1):eabc7409.
Meldrum DR. Female reproductive aging—ovarian and uterine factors. Fertil Steril. 1993;59(1):1–5.
Tilly JL, Sinclair DA. Germline energetics, aging, and female infertility. Cell Metab. 2013;17(6):838–50.
Griffith OW, Chavan AR, Protopapas S, et al. Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc Natl Acad Sci. 2017;114(32):E6566–75.
Kelly RW. Pregnancy maintenance and parturition: the role of prostaglandin in manipulating the immune and inflammatory response. Endocr Rev. 1994;15(5):684–706.
Romero R, Espinoza J, Gonçalves LF, et al. Inflammation in preterm and term labour and delivery. In: Seminars in Fetal and Neonatal Medicine. Elsevier; 2006.
Pontillo A, Girardelli M, Agostinis C, et al. Bacterial LPS differently modulates inflammasome gene expression and IL-1β secretion in trophoblast cells, decidual stromal cells, and decidual endothelial cells. Reprod Sci. 2013;20(5):563–6.
Kaneko Y, Sano M, Seno K, et al. Olive leaf extract (OleaVita) suppresses inflammatory cytokine production and NLRP3 inflammasomes in human placenta. Nutrients. 2019;11(5):970.
Bryant AH, Bevan RJ, Spencer-Harty S, et al. Expression and function of NOD-like receptors by human term gestation-associated tissues. Placenta. 2017;58:25–32.
Romero R, Xu Y, Plazyo O, et al. A role for the inflammasome in spontaneous labor at term. Am J Reprod Immunol. 2018;79(6):e12440.
Wang S, Zheng Y, Li J, et al. Single-cell transcriptomic atlas of primate ovarian aging. Cell. 2020;180(3):585-600.e19.
Romero R, Miranda J, Kusanovic JP, et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med. 2015;43(1):19–36.
Gomez-Lopez N, Romero R, Xu Y, et al. A role for the inflammasome in spontaneous labor at term with acute histologic chorioamnionitis. Reprod Sci. 2017;24(6):934–53.
Gomez-Lopez N, Romero R, Xu Y, et al. Inflammasome assembly in the chorioamniotic membranes during spontaneous labor at term. Am J Reprod Immunol. 2017;77(5):e12648.
Halgunset J, Johnsen H, Kjøllesdal AM, et al. Cytokine levels in amniotic fluid and inflammatory changes in the placenta from normal deliveries at term. Eur J Obstet Gynecol Reprod Biol. 1994;56(3):153–60.
Gomez-Lopez N, Motomura K, Miller D, et al. Inflammasomes: their role in normal and complicated pregnancies. J Immunol. 2019;203(11):2757–69.
Romero R, Miranda J, Chaiworapongsa T, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol. 2014;71(4):330–58.
Gomez-Lopez N, Romero R, Panaitescu B, et al. Inflammasome activation during spontaneous preterm labor with intra-amniotic infection or sterile intra-amniotic inflammation. Am J Reprod Immunol. 2018;80(5):e13049.
Weel IC, Romão-Veiga M, Matias ML, et al. Increased expression of NLRP3 inflammasome in placentas from pregnant women with severe preeclampsia. J Reprod Immunol. 2017;123:40–7.
Liu Z, Zhao X, Shan H, et al. microRNA-520c-3p suppresses NLRP3 inflammasome activation and inflammatory cascade in preeclampsia by downregulating NLRP3. Inflamm Res. 2019;68(8):643–54.
Blevins HM, Xu Y, Biby S, et al. The NLRP3 inflammasome pathway: a review of mechanisms and inhibitors for the treatment of inflammatory diseases. Frontiers in Aging. Neuroscience. 2022;14.
Zahid A, Li B, Kombe AJK, et al. Pharmacological Inhibitors of the NLRP3 Inflammasome. Front Immunol. 2019;10:2538.
Yu J-W, Lee M-S. Mitochondria and the NLRP3 inflammasome: physiological and pathological relevance. Arch Pharm Res. 2016;39(11):1503–18.
Mrityunjaya M, Pavithra V, Neelam R, et al. Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of COVID-19. Front Immunol. 2020;2337.
Tunbridge M, França Gois PH. Vitamin D and the NLRP3 inflammasome. Appl Sci. 2020;10(23):8462.
Lee H, Lim Y. Gamma-tocopherol ameliorates hyperglycemia-induced hepatic inflammation associated with NLRP3 inflammasome in alloxan-induced diabetic mice. Nutr Res Pract. 2019;13(5):377–83.
Pizzicannella J, Fonticoli L, Guarnieri S, et al. Antioxidant ascorbic acid modulates NLRP3 inflammasome in LPS-G treated oral stem cells through NFκB/Caspase-1/IL-1β pathway. Antioxidants. 2021;10(5):797.
Lee SC, Wang SY, Li CC, et al. Anti-inflammatory effect of cinnamaldehyde and linalool from the leaf essential oil of Cinnamomum osmophloeum Kanehira in endotoxin-induced mice. J Food Drug Anal. 2018;26(1):211–20.
Mercola J, Grant WB, Wagner CL. Evidence regarding vitamin D and risk of COVID-19 and its severity. Nutrients. 2020;12(11):3361.
Teshome A, Adane A, Girma B, et al. The impact of vitamin D level on COVID-19 infection: systematic review and meta-analysis. Front Public Health. 2021;9:624559.
Li Y, Yang G, Yang X, et al. Nicotinic acid inhibits NLRP3 inflammasome activation via SIRT1 in vascular endothelial cells. Int Immunopharmacol. 2016;40:211–8.
Zhang P, Tsuchiya K, Kinoshita T, et al. Vitamin B6 prevents IL-1β protein production by inhibiting NLRP3 inflammasome activation. J Biol Chem. 2016;291(47):24517–27.
Ahn H, Lee G-S. Riboflavin, vitamin B2, attenuates NLRP3, NLRC4, AIM2, and non-canonical inflammasomes by the inhibition of caspase-1 activity. Sci Rep. 2020;10(1):1–10.
Maywald M, Wessels I, Rink L. Zinc signals and immunity. Int J Mol Sci. 2017;18(10):2222.
Wessels I, Maywald M, Rink L. Zinc as a gatekeeper of immune function. Nutrients. 2017;9(12):1286.
Jq L, Tian H, Xg Z, et al. Zinc provides neuroprotection by regulating NLRP3 inflammasome through autophagy and ubiquitination in a spinal contusion injury model. CNS Neurosci Ther. 2021;27(4):413–25.
Wu J, Li X, Zhu G, et al. The role of Resveratrol-induced mitophagy/autophagy in peritoneal mesothelial cells inflammatory injury via NLRP3 inflammasome activation triggered by mitochondrial ROS. Exp Cell Res. 2016;341(1):42–53.
Hu JJ, Liu X, Xia S, et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat Immunol. 2020;21(7):736–45.
Lamkanfi M, Mueller JL, Vitari AC, et al. Glyburide inhibits the cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187(1):61–70.
Coll RC, Hill JR, Day CJ, et al. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat Chem Biol. 2019;15(6):556–9.
Juliana C, Fernandes-Alnemri T, Wu J, et al. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J Biol Chem. 2010;285(13):9792–802.
Zhang F, Wang L, Wang J-j, et al. The caspase-1 inhibitor AC-YVAD-CMK attenuates acute gastric injury in mice: involvement of silencing NLRP3 inflammasome activities. Sci Rep. 2016;6(1):24166 2016/04/07.
Wang Y, Yu B, Wang L, et al. Pioglitazone ameliorates glomerular NLRP3 inflammasome activation in apolipoprotein E knockout mice with diabetes mellitus. PLoS ONE. 2017;12(7):e0181248.
Amirian M, Shariat Moghani S, Jafarian F, et al. Combination of pioglitazone and clomiphene citrate versus clomiphene citrate alone for infertile women with the polycystic ovarian syndrome. BMC Womens Health. 2021;21(1):302 2021/08/17.
Tang B, Liu D, Chen L, et al. NLRP3 inflammasome inhibitor MCC950 attenuates primary dysmenorrhea in mice via the NF-κB/COX-2/PG pathway. J Inflamm. 2020;17(1):1–9.
Guo X, Xu X, Li T, et al. NLRP3 inflammasome activation of mast cells by estrogen via the nuclear-initiated signaling pathway contributes to the development of endometriosis. Front Immunol. 2021;3909.
Hibi H, Kato K, Mitsui K, et al. Treatment of oligoasthenozoospermia with tranilast, a mast cell blocker, after long-term administration. Arch Androl. 2002;48(6):451–9.
Author information
Authors and Affiliations
Contributions
E. H., M. B.: conceptualization, supervision, and writing — original draft preparation. H.-R. K.-G., C. A. A., and M. N.: supervision and writing — reviewing and editing. M. A., A. Z., S. N. M., R. K., P. A., and S. E.: made impressive contributions to writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hosseini, E., Kohan-Ghadr, HR., Bazrafkan, M. et al. Rescuing fertility during COVID-19 infection: exploring potential pharmacological and natural therapeutic approaches for comorbidity, by focusing on NLRP3 inflammasome mechanism.. J Assist Reprod Genet 40, 1173–1185 (2023). https://doi.org/10.1007/s10815-023-02768-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-02768-1