Abstract
Purpose
The purpose was to evaluate the effect of intrauterine injection of aBMNC on the endometrial function in patients with refractory Asherman’s syndrome (AS) and/or thin and dysfunctional endometrium (TE).
Study design
This is a prospective, experimental, non-controlled study
Material and methods
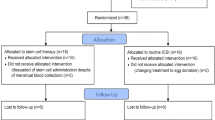
The study was carried out between December 2018 and December 2020 on 20 patients, who were of age < 45 years and had oligo/amenorrhea and primary infertility due to refractory AS and/or TE. One hundred ml BM was extracted. aBMNC cells were separated according to generic volume reduction protocol by using the Cell Separation System SEPAX S-100 table top centrifuge system. We have evaluated CD34+, mononuclear cell (MNC), and total nucleated cell (TNC) counts. The transplantation aBMNC was performed by two intrauterine injections at an interval of one week, transvaginally into the endometrial–myometrial junction by an ovum aspiration needle. Midcyclic endometrial thickness (ET) and gestations after transplantation were evaluated.
Results
The mean TNC, MNC, and CD34+ cells were 11.55 ± 4.7 × 108, 3.85 ± 2.01 × 108, and 7.00 ± 2.88 × 106 at first injection, respectively, and 6.85 ± 2.67 × 108, 2.04 ± 1.11 × 108, and 3.44 ± 1.31 × 106 at second injection, respectively.
The maximum posttransplantation ET was significantly higher than the maximum pretransplantation ET: 2.97 ± 0.48 vs. 5.76 ± 1.19 (mean ± standard deviation, p < 0.01). Twelve patients had frozen-thaw embryo transfers after the study.
In 42% (n = 5 of 12) of the patients, pregnancy was achieved. One of the five patients delivered a healthy baby at term.
Conclusions
Autologous BMNC transplantation may contribute to endometrial function in patients with AS and/or TE.



Similar content being viewed by others
References
Asherman JG. Amenorrhoea traumatica (atretica). BJOG. 1948;55(1):23–30. https://doi.org/10.1111/j.1471-0528.1948.tb07045.x.
Asherman JG. Traumatic intrauterine adhesions. BJOG. 1950;57(6):892–6. https://doi.org/10.1111/j.1471-0528.1950.tb06053.x.
Yu D, Wong YM, Cheong Y, Xia E, Li TC. Asherman syndrome—one century later. Fertil Steril. 2008;89:759–79. https://doi.org/10.1016/j.fertnstert.2008.02.096.
Senturk LM, Erel CT. Thin endometrium in assisted reproductive technology. Curr Opin Obstet Gynecol. 2008;20:221–8. https://doi.org/10.1097/GCO.0b013e328302143c.
Mouhayar Y, Sharara F. Modern management of thin lining. Middle East Fertil Soc J. 2017;22:1–2. https://doi.org/10.1016/j.mefs.2016.09.001.
Gargett CE, Ye L. Endometrial reconstruction from stem cells. Fertil Steril. 2012;98:1120. https://doi.org/10.1016/j.fertnstert.2012.05.004.
Ratajczak MZ, Zuba-Surma EK, Machalinski B, Ratajczak J, Kucia M. Very small embryonic-like (VSEL) stem cells: purification from adult organs, characterization, and biological significance. Stem Cell Rev. 2008;4:89–99. https://doi.org/10.1007/s12015-008-9018-0.
Franz RW, Parks A, Shah KJ, Hankins T, Hartman JF, Wright ML. Use of autologous bone marrow mononuclear cell implantation therapy as a limb salvage procedure in patients with severe peripheral arterial disease. J Vasc Surg. 2009;50:1378–90. https://doi.org/10.1016/j.jvs.2009.07.113.
Yanishi K, Shoji K, Fujioka A, Hori Y, Yukawa A, Matoba S. Impact of therapeutic angiogenesis using autologous bone marrow-derived mononuclear cell implantation in patients with no-option critical limb ischemia. Ann Vasc Dis. 2020;13:13–22. https://doi.org/10.3400/avd.ra.20-00002.
Beltran-Camacho L, Jimenez-Palomares M, Torres-Rojas M, Perez-Segura MC, Serrano A, Sanchez-Gomar I, Antonio Rosal-Vela A, Eslava-Alcon S, Perez-Segura MC, Serrano A, Antequera-González B, Alonso-Piñero JA, González-Rovira A, Extremera-García MJ, Rodriguez-Piñero M, Moreno-Luna R, Larsen MR, Larsen MR, Durán-Ruiz MC. Identification of the initial molecular changes detected in response to circulating angiogenic cellsmediated therapy in critical limb ischemia. Stem Cell Res Ther. 2020;11:106. https://doi.org/10.1186/s13287-020-01591-0.
Nossent AY, Bastiaansen AJ, Peters EA, de Vries MR, Aref Z, Welten SM, de Jager SCA, van der Pouw KTCTM, Quax PHA. CCR7-CCL19/CCL21 axis is essential for effective arteriogenesis in a murine model of hindlimb ischemia. J Am Heart Assoc. 2017;6:e005281. https://doi.org/10.1161/JAHA.116.005281.
Lin RZ, Lee CN, Moreno-Luna R, Neumeyer J, Piekarski B, Zhou P, Moses MA, Sachdev M, Pu WT, Emani S, Melero-Martin JM. Host non-inflammatory neutrophils mediate the engraftment of bioengineered vascular networks. Nat Biomed Eng. 2017;1:0081. https://doi.org/10.1038/s41551-017-0081.
Seignez C, Phillipson M. The multitasking neutrophils and their involvement in angiogenesis. Curr Opin Hematol. 2017;24:3–8. https://doi.org/10.1097/MOH.0000000000000300.
Korbling M, Estrov Z. Adult stem cells for tissue repair—a new therapeutic concept? N Engl J Med. 2003;349:570–82. https://doi.org/10.1056/NEJMra022361.
Du HL, Taylor HS. Stem cells and reproduction. Curr Opin Obstet Gynecol. 2010;22:235–41. https://doi.org/10.1097/GCO.0b013e328338c152.
Du H, Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells. 2007;25:2082–6. https://doi.org/10.1634/stemcells.2006-0828.
Cervello I, Martinez-Conejero JA, Horcajadas JA, Pellicer A, Simo’n C. Identification, characterization and co-localization of label-retaining cell population in mouse endometrium with typical undifferentiated markers. Hum Reprod. 2007;22:45–51. https://doi.org/10.1093/humrep/del332.
Mints M, Jansson M, Sadeghi B, Westgren M, Uzunel M, Hassan M, Palmblad J. Endometrial endothelial cells are derived from donor stem cells in a bone marrow transplant recipient. Hum Reprod. 143. https://doi.org/10.1093/humrep/dem342.
Du H, Taylor HS. Stem cells and female reproduction. Reprod Sci. 2009;16:126–39. https://doi.org/10.1177/1933719108329956.
Du H, Naqvi H, Taylor HS. Ischemia/reperfusion injury promotes and granulocytecolony stimulating factor inhibits migration of bone marrow-derived stem cells to endometrium. Stem Cells Dev. 2012;21:3324–31. https://doi.org/10.1089/scd.2011.0193.
Morelli SS, Rameshwar P, Goldsmith LT. Experimental evidence for bone marrow as a source of nonhematopoietic endometrial stromal and epithelial compartment cells in a murine model. Biol Reprod. 2013;89:7. https://doi.org/10.1095/biolreprod.113.107987.
Alawadhi F, Du H, Cakmak H, Taylor HS. Bone marrow-derived stem cell (BMDSC) transplantation improves fertility in a murine model of Asherman’s syndrome. PLoS One. 2014;9:e96662. https://doi.org/10.1371/journal.pone.0096662.
Kilic S, Yuksel B, Pinarli F, Albayrak A, Boztok B, DelibasiT. Effect of stem cell application on Asherman syndrome, an experimental rat model. J Assist Reprod Genet. 2014;31:975–82. https://doi.org/10.1007/s10815-014-0268-2.
Taylor HS. Endometrial cells derived fromdonor stem cells in bone marrow transplant recipients. JAMA. 2004;292:81–5. https://doi.org/10.1001/jama.292.1.81.
Nagori CB, Panchal SY, Patel H. Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient of severe Asherman’s syndrome. J Hum Reprod Sci. 2011;4:43–8. https://doi.org/10.4103/0974-1208.82360.
Gargett CE, Healy DL. Generating receptive endometrium in Asherman's syndrome. J Hum Reprod Sci. 2011;4:49–52.
Santamaria X, Cabanillas S, Cervello I, Arbona C, Raga F, Ferro J, Palmero J, Remohi J, Pellicer A, Simon C. Autologous cell therapy with CD133 bonemarrow-derived stem cells for refractory Asherman’s syndrome andendometrial atrophy: a pilot cohort study. Hum Reprod. 2016;31(5):1087–96. https://doi.org/10.1093/humrep/dew042.
Singh N, Mohanty S, Seth T, Shankar M, Bhaskaran S, Dharmendra S. Autologous stem cell transplantation in refractory Asherman’s syndrome: a novel cell-based therapy. J Hum Reprod Sci. 2014;7:93–8. https://doi.org/10.4103/0974-1208.138864.
Singh N, Shekhar B, Mohanty S, Kumar S, Seth T, Girish B. Autologous bone marrow derived stem cell therapy for Asherman's syndrome and endometrial atrophy: a 5-year follow-up study. J Hum Reprod Sci. 2020;13:31–7. https://doi.org/10.4103/jhrs.JHRS_64_19.
Benor A, Gay A, De Cherney A. An update on stem cell therapy for Asherman syndrome. J Asist Reprod Genet. 2020;37:1511–29. https://doi.org/10.1007/s10815-020-01801-x.
Gharibeh N, Aghebati-Maleki L, Madani J, Pourakbari R, Yousefi M, Javad AH. Cell-based therapy in thin endometrium and Asherman syndrome. Stem Cell Res Ther. 2022;13:33. https://doi.org/10.1186/s13287-021-02698-832.
March C, Israel R, March A. Hysteroscopic management of intrauterine adhesions. Am J Obstet Gynecol. 1978;11:653–7. https://doi.org/10.1016/0002-9378(78)90322-8.
Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther. 2009;17:939–46. https://doi.org/10.1186/s13287-02102698-8.
Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:31–24. https://doi.org/10.1002/path.2469.
Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110(4):624–37. https://doi.org/10.1161/CIRCRESAHA.111.243386.
Wei W, Li L, Lin D, Zhong-Jing W, Jing-Jian D, Xiao-Yu L, Ting J, Li W, Hong-Xiang W, Hong M, Shi Z. Autologous bone marrow mononuclear cell transplantation therapy ımproved symptoms in patients with refractory diabetic sensorimotor polyneuropathy via the mechanisms of paracrine and ımmunomodulation: a controlled study. Cell Transplantation. 2020;29:1–11. https://doi.org/10.1177/0963689720949258.
Kwee BJ, Budina E, Najibi AJ, Mooney DJ. CD4 T-cells regulate angiogenesis and myogenesis. Biomaterials. 2018;178:109–21. https://doi.org/10.1016/j.biomaterials.2018.06.003.
Dehkordi AN, Mirahmadi Babaheydari FM, Chehelgerdi M, Dehkordi SR. Skin tissue engineering: wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res Ther. 2019;29(1):111. https://doi.org/10.1186/s13287-019-1212-2.
Barash A, Dekel N, Fieldust S, Segal I, Schechtman E, Granot I. Local injury to the endometrium doubles the incidence of successful pregnancies in patients undergoing in vitro fertilization. Fertil Steril. 2003;79:1317–22. https://doi.org/10.1016/s0015-0282(03)00345-5.
Zhou L, Li R, Wang R, Huang H, Zhong BA. Local injury to the endometrium in controlled ovarian hyperstimulation cycles improves implantation rates. Fertil Steril. 2008;89:1166–76. https://doi.org/10.1016/j.fertnstert.2007.05.064.
Wysoczynki M, Khan A, Bolli B. New paradigms in cell therapy: repeated dosing, intravenous delivery, immunomodulatory actions, and new cell types. Circ Res. 2018;123(2):138–58. https://doi.org/10.1161/CIRCRESAHA.118.313251.
Bolli R. Repeated cell therapy: a paradigm shift whose time has come. Circ Res. 2017;120:1072–4. https://doi.org/10.1161/CIRCRESAHA.117.310710.
Tokita Y, Tang XL, Li Q, Wysoczynski M, Hong KU, Nakamura S, Wu WJ, Xie W, Li D, Hunt G, Ou Q, Stowers H, Bolli R. Repeated administrations of cardiac progenitor cells are markedly more effective than a single administration: a new paradigm in cell therapy. Circ Res. 2016;119:635–51. https://doi.org/10.1161/CIRCRESAHA.116.308937.
Guo Y, Wysoczynski M, Nong Y, Tomlin A, Zhu X, Gumpert AM, Nasr M, Muthusamy S, Li H, Book M, Khan A, Hong KU, Li Q, Bolli R. Repeated doses of cardiac mesenchymal cells are therapeutically superior to a single dose in mice with old myocardial infarction. Basic Res Cardiol. 2017;112:18. https://doi.org/10.1007/s00395-017-0606-5.
Jarocha D, Zuba-Surma E, Majka M. Dimethyl sulfoxide (DMSO) increases percentage of CXCR4(+) hematopoietic stem/progenitor cells, their responsiveness to an SDF-1 gradient, homing capacities, and survival. Cell Transplant. 2016;25(7):1247–57. https://doi.org/10.3727/096368915X689424.
Huang J, Li Q, Yuan X, Liu Q, Zhang W, Li P. Intrauterine infusion of clinically graded human umbilical cord-derived mesenchymal stem cells for the treatment of poor healing after uterine injury: a phase I clinical trial. Stem Cell Res Ther. 2022;13:85. https://doi.org/10.1186/s13287-022-02756-9.
Acknowledgments
We thank Professor Erdal Karaoz (PhD) for his comments on previous and final versions of the manuscript and Ayşenur Kocaoglu and Beril Mensure Karakan, for their technical asistance.
Funding
Partial financial support (for equipment and supplies only) was received from Yeniyüzyil University, Gaziosmanpasa Hospital.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The study was conducted by Gurkan Arikan. The transplantations of aBMNC were performed by Gurkan Arikan, Volkan Turan, and Meryem Kurekeken. The BM aspirations were performed by Hasan Sami Göksoy. The Cell laboratory was run by Zeynep Dogusan. Statistical analyses was performed by Volkan Turan and Meryem Kurekeken. All authors made substantial contributions to the conception and design of the work or to the acquisition, analysis, and interpretation of the data. The first draft of the manuscript was written by Gurkan Arikan and Volkan Turan, and all authors commented on previous versions of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval, consent to participate, and consent to publish
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional ethics committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Bioethics Committee of the Medical University of Yeniyüzyil University (TRN 047, 26.12.2018). Informed consents for participation and for publication of the data were obtained from all patients.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arikan, G., Turan, V., Kurekeken, M. et al. Autologous bone marrow-derived nucleated cell (aBMNC) transplantation improves endometrial function in patients with refractory Asherman’s syndrome or with thin and dysfunctional endometrium. J Assist Reprod Genet 40, 1163–1171 (2023). https://doi.org/10.1007/s10815-023-02727-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-02727-w