Abstract
Purpose
To compare aneuploidy rates in early aborted tissues or blastocysts between in vitro fertilization (IVF) cycles after the gonadotropin-releasing hormone (GnRH) antagonist (GnRH-ant) protocol or the GnRH agonist (GnRH-a) long protocol.
Methods
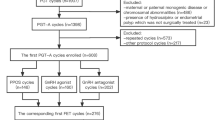
This was a retrospective cohort study from a university-affiliated fertility center. In total, 550 early miscarriage patients who conceived through IVF/intracytoplasmic sperm injection (ICSI) after receiving the GnRH-ant or GnRH-a long protocol were analyzed to compare aneuploidy rates in early aborted tissues. To compare aneuploidy rates in blastocysts, 404 preimplantation genetic testing for aneuploidy (PGT-A) cycles with the GnRH-ant protocol or GnRH-a long protocol were also analyzed.
Results
For early miscarriage patients who conceived through IVF/ICSI, compared to the GnRH-a long protocol group, the GnRH-ant protocol group had a significantly higher rate of aneuploidy in early aborted tissues (48.51% vs. 64.19%). Regarding PGT-A cycles, the rate of blastocyst aneuploidy was significantly higher in the GnRH-ant protocol group than the GnRH-a long protocol group (39.69% vs. 52.27%). After stratification and multiple linear regression, the GnRH-ant regimen remained significantly associated with an increased risk of aneuploidy in early aborted tissues and blastocysts [OR (95% CI) 1.81 (1.21, 2.71), OR (95% CI) 1.65 (1.13, 2.42)]. Furthermore, the blastocyst aneuploidy rate in the GnRH-ant protocol group was significantly higher but only in young and normal ovarian responders [OR (95% CI) 5.07 (1.99, 12.92)].
Conclusion
Compared to the GnRH-a long protocol, the GnRH-ant protocol is associated with a higher aneuploidy rate in early aborted tissues and blastocysts. These results should be confirmed in a multicenter, randomized controlled trial.
Similar content being viewed by others
Data availability
All datasets generated for this study are included in the article/Supplementary Material.
References
Gruhn JR, Zielinska AP, Shukla V, Blanshard R, Capalbo A, Cimadomo D, Nikiforov D, Chan AC, Newnham LJ, Vogel I, et al. Chromosome errors in human eggs shape natural fertility over reproductive life span. Science (New York, NY). 2019;365(6460):1466–9.
Rai R, Regan L. Recurrent miscarriage. Lancet (London, England). 2006;368(9535):601–11.
Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, Scott RT Jr. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101(3):656-663.e651.
Haddad G, Deng M, Wang CT, Witz C, Williams D, Griffith J, Skorupski J, Gill J, Wang WH. Assessment of aneuploidy formation in human blastocysts resulting from donated eggs and the necessity of the embryos for aneuploidy screening. J Assist Reprod Genet. 2015;32(6):999–1006.
Mikwar M, MacFarlane AJ, Marchetti F. Mechanisms of oocyte aneuploidy associated with advanced maternal age. Mutat Res. 2020;785:108320.
Derrick R, Hickman C, Oliana O, Wilkinson T, Gwinnett D, Whyte LB, Carby A, Lavery S. Perivitelline threads associated with fragments in human cleavage stage embryos observed through time-lapse microscopy. Reprod Biomed Online. 2017;35(6):640–5.
Macklon NS, Stouffer RL, Giudice LC, Fauser BC. The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev. 2006;27(2):170–207.
Li G, Wu Y, Niu W, Xu J, Hu L, Shi H, Sun Y. Analysis of the number of euploid embryos in preimplantation genetic testing cycles with early-follicular phase long-acting gonadotropin-releasing hormone agonist long protocol. Front Endocrinol. 2020;11:424.
Cascales A, Lledo B, Ortiz JA, Morales R, Ten J, Llacer J, Bernabeu R. Effect of ovarian stimulation on embryo aneuploidy and mosaicism rate. Syst Biol Reprod Med. 2021;67(1):42–9.
Ubaldi FM, Capalbo A, Vaiarelli A, Cimadomo D, Colamaria S, Alviggi C, Trabucco E, Venturella R, Vajta G, Rienzi L. Follicular versus luteal phase ovarian stimulation during the same menstrual cycle (DuoStim) in a reduced ovarian reserve population results in a similar euploid blastocyst formation rate: new insight in ovarian reserve exploitation. Fertil Steril. 2016;105(6):1488-1495.e1481.
Yang R, Guan Y, Perrot V, Ma J, Li R. Comparison of the long-acting GnRH agonist follicular protocol with the GnRH antagonist protocol in women undergoing in vitro fertilization: a systematic review and meta-analysis. Adv Ther 2021.
Wang R, Lin S, Wang Y, Qian W, Zhou L. Comparisons of GnRH antagonist protocol versus GnRH agonist long protocol in patients with normal ovarian reserve: a systematic review and meta-analysis. PLoS ONE. 2017;12(4):e0175985.
Xiao JS, Su CM, Zeng XT. Comparisons of GnRH antagonist versus GnRH agonist protocol in supposed normal ovarian responders undergoing IVF: a systematic review and meta-analysis. PLoS ONE. 2014;9(9):e106854.
Lambalk CB, Banga FR, Huirne JA, Toftager M, Pinborg A, Homburg R, van der Veen F, van Wely M. GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Hum Reprod Update. 2017;23(5):560–79.
Bahceci M, Ulug U, Sismanoglu A, Tosun S, Cengiz B. Early pregnancy loss rates were different among singleton gestations conceived by ICSI using GnRH agonist and antagonist. J Assist Reprod Genet. 2009;26(4):227–9.
Maldonado LG, Franco JG Jr, Setti AS, Iaconelli A Jr, Borges E Jr. Cost-effectiveness comparison between pituitary down-regulation with a gonadotropin-releasing hormone agonist short regimen on alternate days and an antagonist protocol for assisted fertilization treatments. Fertil Steril. 2013;99(6):1615–22.
Hu L, Du J, Lv H, Zhao J, Chen M, Wang Y, Wu F, Liu F, Chen X, Zhang J, et al. Influencing factors of pregnancy loss and survival probability of clinical pregnancies conceived through assisted reproductive technology. Reprod Biol Endocrinol. 2018;16(1):74.
Lv H, Diao F, Du J, Chen T, Meng Q, Ling X, Li H, Song C, Xi Q, Jiang Y et al. Assisted reproductive technology and birth defects in a Chinese birth cohort study. Lancet Reg Health - West Pac. 2021;7.
Colley E, Hamilton S, Smith P, Morgan NV, Coomarasamy A, Allen S. Potential genetic causes of miscarriage in euploid pregnancies: a systematic review. Hum Reprod Update. 2019;25(4):452–72.
Hartman RJ, Rasmussen SA, Botto LD, Riehle-Colarusso T, Martin CL, Cragan JD, Shin M, Correa A. The contribution of chromosomal abnormalities to congenital heart defects: a population-based study. Pediatr Cardiol. 2011;32(8):1147–57.
Davis AR, Horvath SK, Castano PM. Trends in gestational age at time of surgical abortion for fetal aneuploidy and structural abnormalities. Am J Obstet Gynecol. 2017;216(3):278 e271-278 e275.
Ozgur K, Berkkanoglu M, Bulut H, Humaidan P, Coetzee K. Perinatal outcomes after fresh versus vitrified-warmed blastocyst transfer: retrospective analysis. Fertil Steril. 2015;104(4):899-907.e893.
Thorne J, Loza A, Kaye L, Nulsen J, Benadiva C, Grow D, Engmann L. Euploidy rates between cycles triggered with gonadotropin-releasing hormone agonist and human chorionic gonadotropin. Fertil Steril. 2019;112(2):258–65.
Spielmann H, Vogel R. Genotoxic and embryotoxic effects of gonadotropin hyperstimulated ovulation on murine oocytes, preimplantation embryos and term fetuses. Ann Ist Super Sanita. 1993;29(1):35–9.
Roberts R, Iatropoulou A, Ciantar D, Stark J, Becker DL, Franks S, Hardy K. Follicle-stimulating hormone affects metaphase I chromosome alignment and increases aneuploidy in mouse oocytes matured in vitro. Biol Reprod. 2005;72(1):107–18.
McCulloh DH, Alikani M, Norian J, Kolb B, Arbones JM, Munne S. Controlled ovarian hyperstimulation (COH) parameters associated with euploidy rates in donor oocytes. Eur J Med Genet. 2019;62(8):103707.
Sachdeva K, Upadhyay D, Discutido R, Varghese MM, Albuz F, Almekosh R, Bouhafs L, Solkar S, Stevikova M, Peramo B. Low gonadotropin dosage reduces aneuploidy in human preimplantation embryos: first clinical study in a UAE population. Genet Test Mol Biomarkers. 2018;22(10):630–4.
Baart EB, Martini E, Eijkemans MJ, Van Opstal D, Beckers NG, Verhoeff A, Macklon NS, Fauser BC. Milder ovarian stimulation for in-vitro fertilization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum Reprod (Oxford, England). 2007;22(4):980–8.
Katz-Jaffe MG, Trounson AO, Cram DS. Chromosome 21 mosaic human preimplantation embryos predominantly arise from diploid conceptions. Fertil Steril. 2005;84(3):634–43.
Wu Q, Li H, Zhu Y, Jiang W, Lu J, Wei D, Yan J, Chen ZJ. Dosage of exogenous gonadotropins is not associated with blastocyst aneuploidy or live-birth rates in PGS cycles in Chinese women. Hum Reprod. 2018;33(10):1875–82.
Barash OO, Hinckley MD, Rosenbluth EM, Ivani KA, Weckstein LN. High gonadotropin dosage does not affect euploidy and pregnancy rates in IVF PGS cycles with single embryo transfer. Hum Reprod. 2017;32(11):2209–17.
Sekhon L, Shaia K, Santistevan A, Cohn KH, Lee JA, Beim PY, Copperman AB. The cumulative dose of gonadotropins used for controlled ovarian stimulation does not influence the odds of embryonic aneuploidy in patients with normal ovarian response. J Assist Reprod Genet. 2017;34(6):749–58.
Irani M, Canon C, Robles A, Maddy B, Gunnala V, Qin X, Zhang C, Xu K, Rosenwaks Z. No effect of ovarian stimulation and oocyte yield on euploidy and live birth rates: an analysis of 12 298 trophectoderm biopsies. Hum Reprod (Oxford, England). 2020;35(5):1082–9.
Munne S, Alikani M, Ribustello L, Colls P, Martinez-Ortiz PA, McCulloh DH, Referring Physician G. Euploidy rates in donor egg cycles significantly differ between fertility centers. Hum Reprod. 2017;32(4):743–9.
Yang R, Guan Y, Perrot V, Ma J, Li R. Comparison of the long-acting GnRH agonist follicular protocol with the GnRH antagonist protocol in women undergoing in vitro fertilization: a systematic review and meta-analysis. Adv Ther. 2021;38(5):2027–37.
Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod. 1996;11(10):2217–22.
Zeng HT, Ren Z, Yeung WS, Shu YM, Xu YW, Zhuang GL, Liang XY. Low mitochondrial DNA and ATP contents contribute to the absence of birefringent spindle imaged with PolScope in in vitro matured human oocytes. Hum Reprod. 2007;22(6):1681–6.
Zhang X, Wu XQ, Lu S, Guo YL, Ma X. Deficit of mitochondria-derived ATP during oxidative stress impairs mouse MII oocyte spindles. Cell Res. 2006;16(10):841–50.
Lee M, Ahn JI, Lee AR, Ko DW, Yang WS, Lee G, Ahn JY, Lim JM. Adverse effect of superovulation treatment on maturation, function and ultrastructural integrity of murine oocytes. Mol Cells. 2017;40(8):558–66.
Xie JK, Wang Q, Zhang TT, Yin S, Zhang CL, Ge ZJ. Repeated superovulation may affect mitochondrial functions of cumulus cells in mice. Sci Rep. 2016;6:31368.
Dong G, Guo Y, Cao H, Zhou T, Zhou Z, Sha J, Guo X, Zhu H. Long-term effects of repeated superovulation on ovarian structure and function in rhesus monkeys. Fertil Steril. 2014;102(5):1452-1457 e1451.
Xiao P, Nie J, Wang X, Lu K, Lu S, Liang X. Melatonin alleviates the deterioration of oocytes from mice subjected to repeated superovulation. J Cell Physiol. 2019;234(8):13413–22.
Al-Zubaidi U, Adhikari D, Cinar O, Zhang QH, Yuen WS, Murphy MP, Rombauts L, Robker RL, Carroll J. Mitochondria-targeted therapeutics, MitoQ and BGP-15, reverse aging-associated meiotic spindle defects in mouse and human oocytes. Hum Reprod. 2021;36(3):771–84.
Perkins AT, Das TM, Panzera LC, Bickel SE. Oxidative stress in oocytes during midprophase induces premature loss of cohesion and chromosome segregation errors. Proc Natl Acad Sci U S A. 2016;113(44):E6823–30.
Acknowledgements
The authors gratefully acknowledge the patients who participated in this study.
Funding
This work was supported by the National Natural Science Foundation of China (81671463) and the Shaanxi Province Key R & D Program General Project (2021SF-012).
Author information
Authors and Affiliations
Contributions
Jun Wang collected the data and prepared all the tables and wrote the main manuscript, Jing Zhang and Nan Zhao collected the data, Shuqiang Chen searched the literature and designed the study, and Xiaohong Wang revised the manuscript. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the ethics committee of the Tang Du Hospital of the Air Force Military Medical University, China (K202108-09). All patients provided written informed consent.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, J., Zhang, J., Zhao, N. et al. The effect of ovarian stimulation on aneuploidy of early aborted tissues and preimplantation blastocysts: comparison of the GnRH agonist long protocol with the GnRH antagonist protocol. J Assist Reprod Genet 39, 1927–1936 (2022). https://doi.org/10.1007/s10815-022-02557-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02557-2