Abstract
Introduction
To evaluate the effectiveness and safety of long-acting GnRH agonist follicular and GnRH antagonist protocols among women undergoing in vitro fertilization (IVF) using data published in both English-language and Chinese studies.
Methods
We systematically searched the PubMed, Embase, Cochrane, CNKI, and Wanfang databases up to March 2019 for studies comparing long-acting GnRH agonist follicular and GnRH antagonist protocols in women undergoing IVF. The primary outcome was live birth rate; secondary outcomes were clinical pregnancy rate and implantation rate; safety outcomes were ovarian hyperstimulation syndrome (OHSS) and miscarriage rate in fresh cycle. Statistical analysis was done using R software. The study protocol was registered with PROSPERO (CRD42019139396).
Results
In 11 studies that met the inclusion criteria, 1994 women belonged to the long-acting GnRH agonist follicular protocol group and 1678 to the GnRH antagonist protocol group. Live birth rate (relative risk (RR) 1.61; 95% confidence interval (CI) 1.27, 2.05; P < 0.001), clinical pregnancy rate (RR 1.44; 95% CI 1.32, 1.58; P < 0.001), and implantation rate (RR 1.58; 95% CI 1.44, 1.73; P = 0.001) were higher in the long-acting GnRH agonist follicular protocol compared with the antagonist protocol group. There was no difference in miscarriage rate (RR 0.98; 95% CI 0.58, 1.64; P = 0.98) between the long-acting GnRH agonist follicular and antagonist protocols. However, OHSS rate (RR 1.63; 95% CI 1.15, 2.32; P = 0.0058) was lower in the GnRH antagonist protocol compared to the long-acting GnRH agonist protocol group.
Conclusion
The long-acting GnRH agonist follicular protocol was beneficial in improving live birth rate, clinical pregnancy rate, and implantation rate whereas the incidence of OHSS was significantly lower in women undergoing the GnRH antagonist protocol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The best protocol among long-acting GnRH-agonist follicular protocol and GnRH-antagonist protocols for in vitro fertilization is widely debated in the literature, and the optimal protocol remains inconclusive due to several confounders including variation in study population. |
In this meta-analysis, we evaluated the effectiveness and safety of long-acting GnRH-agonist follicular and antagonist protocols using the published data from English and Chinese studies. |
Live birth rate, clinical pregnancy rate and implantation rate were significantly higher in the long-acting GnRH-agonist follicular protocol compared with the antagonist protocol group. |
Ovarian hyperstimulation syndrome rate was significantly lower in the GnRH-antagonist protocol and there was no difference in miscarriage rate. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12967709.
Introduction
The gonadotropin-releasing hormone (GnRH) agonist and the GnRH antagonist protocols are well-established methods for controlled ovarian hyperstimulation among patients who are undergoing assisted reproductive technology (ART) [1]. Since the advent of GnRH agonists in the 1980s to prevent premature luteinizing hormone (LH) outpouring, thereby increasing the number of retrieved oocytes and pregnancy rates, GnRH agonist protocols have become the gold standard for in vitro fertilization (IVF) [2, 3]. The mechanism of action with sustained treatment of GnRH-agonist involves induction of both the endogenous LH surge and ovulation, and cause complete refractoriness of the pituitary to GnRH action in the later stage which may lead to prevention of premature LH surge [4]. Prolonged downregulation achieved by the GnRH agonist protocol may increase the endometrial receptivity of women undergoing IVF treatment leading to better reproductive outcomes [5,6,7]. A recent systematic review and meta-analysis emphasizes the long-acting GnRH agonist protocol as the first-choice treatment with increased ongoing pregnancy rate compared with the GnRH antagonist protocol [8]. Though the long-acting GnRH agonist protocol is associated with ovarian hyperstimulation syndrome (OHSS) or other side effects, a recent study by Van den Wijngaard et al. evaluating patients’ preferences using discrete choice analysis showed that the majority of patients preferred a long-acting GnRH agonist protocol favoring increased pregnancy rate compared to an antagonist protocol [2]. Moreover, it can shorten the time to live birth in fresh transfer cycle relative to frozen transfer cycle. A Cochrane review by Albuquerque et al. highlights the advantages of the long-acting GnRH agonist protocol among the other types of GnRH agonist ovarian-stimulating protocols [9]. In a recent study, Geng et al. demonstrated the positive effect of the long-acting GnRH agonist follicular protocol on reproductive outcome by increasing the endometrial receptivity of patients undergoing IVF compared to results with the GnRH antagonist protocol [5]. Furthermore, in the long-acting GnRH agonist follicular protocol, a full single dose of 3.75 mg long-acting GnRH agonist was administered during early follicular phase (ca. 1–5 days) of the menstrual cycle; which is different from the traditional long-acting GnRH agonist protocol where GnRH agonist usually starts in the mid-luteal phase of the menstrual cycle.
Randomized controlled trials (RCTs) and meta-analysis comparing long-acting GnRH agonist and GnRH antagonist protocols on pregnancy rate and live birth rate have resulted mixed findings. For example, a systematic review reported no difference in clinical pregnancy rate and live birth rates with the GnRH antagonist protocol compared with the long-acting GnRH agonist protocol; however, the incidence of OHSS was lower in long-acting GnRH agonist protocol [10]. Another study reported equivalent live birth rate with both protocols [11]. The best protocol for IVF is widely debated in the literature and the optimal protocol remains inconclusive because of several confounders including variation in study population, variation in treatment arms apart from agonist and antagonists, and variation in stimulation strategies [1]. In China, different GnRH agonist protocols are used flexibly and long-acting GnRH agonist follicular protocols have been used in increasing number of IVF centers in recent years. Of note, long-acting GnRH agonist follicular protocols are widely used in China but the results of these studies, being published in Chinese, are often excluded in the meta-analyses published in other countries thus leading to publication bias [8]. Moreover, till date, no published meta-analysis exists evaluating the effectiveness of the long-acting GnRH agonist follicular protocol compared with the GnRH antagonist protocols to our knowledge. Hence, in this meta-analysis, we evaluated the effectiveness and safety of long-acting GnRH agonist follicular and antagonist protocols using the published data from English and Chinese studies and hope the result will help with IVF clinical practice.
Methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [12].
This article is based on previously conducted studies and the authors did not conduct any experiments that included human participants or animals.
Search Strategy and Participants
A literature search was performed in PubMed, Embase, Cochrane, CNKI, and Wanfang for articles published up to 1 March 2019 using the following search strings: [(GnRH a or GnRHa or GnRH agonist or gonadotropin-releasing hormone agonist or gonadorelin or triptorelin or goserelin or leuprorelin or nafarelin or alarelin or histrelin) and (agonist protocol) and (GnRH ant or GnRH antagonist or gonadotropin-releasing hormone antagonist or cetrorelix or ganirelix or teverelix) and (antagonist protocol)]. The corresponding Chinese search string is provided in Supplementary Table 1.
Duplicates were removed and all the studies were screened as per the inclusion criteria by two independent reviewers after reaching consensus on the eligibility of the study.
Inclusion and Exclusion Criteria
Eligible studies were RCTs, prospective non-randomized studies, observational, cohort, and retrospective studies comparing the long-acting GnRH agonist follicular protocol with GnRH antagonist protocol and studies reporting live birth rate, clinical pregnancy rate, implantation rate, miscarriage, or OHSS. The long-acting GnRH agonist follicular protocol: a full single dose of 3.75 mg long-acting GnRH agonist was administered in the early follicular phase (ca. 1–5 days) of the menstrual cycle; ovarian stimulation was started if pituitary downregulation was established (mostly 28 days after GnRH agonist administration) until trigger. GnRH antagonist protocol: ovarian stimulation was started in the early follicular phase (ca. 1–5 days) of the menstrual cycle, a few days after GnRH antagonist was administered daily until ovulation was triggered.
Studies with the following characteristics were excluded: meta-analysis, systematic literature reviews, narrative reviews, case reports, conference proceedings, results not reporting desired objective and outcomes of interest, studies reporting combination therapy of long-acting GnRH agonist follicular and GnRH antagonist protocols, frozen-thawed embryo transfer study, animal study, and non-English articles (for PubMed, Embase, and Cochrane), articles with calculation errors in the reported results were also excluded. The study protocol was registered in PROSPERO (CRD42019139396).
Data Extraction and Quality Assessment
Two independent reviewers extracted data such as author, year of publication, title, study design, demographics of the study population and outcomes of interest from included studies into standardized MS Office Excel sheet. The methodological quality of eligible RCTs and observational studies was assessed using the Jadad scale [13] and Newcastle-Ottawa scale respectively. The publication bias was evaluated using funnel plots for live birth rate, clinical pregnancy rate, and implantation rate.
Study Outcomes
The primary outcome of the study was live birth rate (LBR); secondary outcomes were clinical pregnancy rate and implantation rate, presented as incidence. Safety outcomes like miscarriage and OHSS were presented as proportions.
Statistical Analysis
All the data management, relevancy and duplication removal, and assessment of eligibility as per PRISMA guidelines were performed using Microsoft Excel. The statistical data analysis was performed after completion of validation and quality checks using R statistical software. Descriptive statistics were used to analyze the baseline parameters and all continuous variables were presented as means, medians, and standard deviations. For analysis, all comparisons of LBR, pregnancy rate, implantation rate, and OHSS rate were reported as relative risk (RR) with 95% confidence interval (CI) for clinical outcomes and presented as Forest plots. Even though some papers have reported moderate or severe OHSS, some reported total OHSS, all papers were combined for analysis as OHSS rate. RR was calculated by the metaphor package using R software. Heterogeneity among the studies was determined via Cochrane’s Q and I2 statistics. A fixed effects (FE) model was used when heterogeneity was low (I2 < 50%) and a random effects (RE) model was used when I2 was greater than 50%. If the P value for heterogeneity was < 0.05 or I2 is > 50%, the heterogeneity was considered statistically significant.
Results
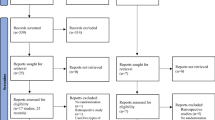
The search identified 5331 hits. Following screening, 11 articles were included in the comparison of the long-acting GnRH agonist follicular protocol with GnRH antagonist protocol for analysis (Fig. 1). Among 11 studies included, 10 were observational studies (9 retrospective study; 1 prospective study) and one was a RCT (Supplementary table 2). There were nine Chinese and two English articles included in the analysis. The number of women in the agonist and antagonist arms were 1994 and 1678, respectively; the mean age in both the groups was 30.9 years. The proportions of normal ovarian responders, polycystic ovary syndrome (PCOS), and poor responders in each group were 69.2%, 27.1%, and 3.8%, respectively, in the agonist group and 42.5%, 46.5%, and 11.0%, respectively, in the antagonist group.
Quality Assessment and Publication Bias
Publication Bias
Publication bias of LBR, clinical pregnancy rate, and implementation rate depicted by funnel plots showed relatively lesser publication bias among the included studies for the long-acting GnRH agonist follicular protocol compared with the antagonist protocol. The funnel plot asymmetry for LBR (P = 0.35), clinical pregnancy rate (P = 0.49), and implantation rate (P = 0.75) was not statistically significant (Supplementary Fig. 1).
Primary Outcomes
Live Birth Rate
Of the 11 studies, only two reported live birth rate with a range of 45.8–46.6% in the agonist group and 21.2–29.7% in the antagonist group. Live birth rate was significantly higher in the long-acting follicular agonist group compared to the antagonist group (RR 1.61; 95% CI 1.27, 2.05) with the FE model, I2 = 0%, P = < 0.001 (Fig. 2).
Secondary Outcomes
Clinical Pregnancy Rate
All 11 studies [5, 14,15,16,17,18,19,20,21,22,23] provided data on clinical pregnancy rate, which varied from 40.0% to 76.9% in the long-acting follicular agonist and 27.3% to 63.2% in the antagonist protocols. Clinical pregnancy rate was significantly higher in the long-acting follicular agonist group compared to antagonist (RR 1.44; 95% CI 1.32, 1.58), P < 0.001 with the FE model, I2 = 0% (Fig. 3).
Implantation Rate
Six studies [5, 15, 19, 20, 22, 23] reported implantation rate, which varied from 33.33% to 61.4% in the long-acting GnRH agonist follicular group and 20.76% to 38.6% in the antagonist group. Analysis (FE model, I2 = 0%, P < 0.001) showed significantly a higher implantation rate among the women using the long-acting follicular agonist protocol compared to the antagonist protocol (RR 1.58; 95% CI 1.44, 1.73; P < 0.001) (Supplementary Fig. 2).
Miscarriage Rate
Among the eight [14,15,16, 18, 20,21,22,23] studies reporting miscarriage rate, the range in the long-acting GnRH agonist follicular was 5.0–22.2% and 0.00–18% in the antagonist protocol. There was no significant difference between the antagonist treatment group and long-acting follicular agonist group in the miscarriage rate with the FE model, (I2 = 0%, n = 8 studies) (RR 0.98; 95% CI 0.58, 1.64), P = 0.98 (Supplementary Fig. 3).
OHSS Rate
Among the seven studies [5, 14, 18,19,20,21, 23] that reported OHSS rate, three [5, 18, 21] reported total OHSS rate, three [19, 20, 23] reported moderate and severe OHSS rate, and one [14] reported severe OHSS rate. We combined them to analyze the OHSS rate, so the result was just a trend. In the long-acting GnRH agonist follicular protocol group, OHSS rate varied from 3.1% to 46.2%, whereas in the antagonist protocol the rate varied from 2.0% to 21.1%. The antagonist treatment showed a significantly lower OHSS rate compared to the long-acting follicular agonist protocol in analysis with the FE model (I2 = 0%, RR 1.63; 95% CI 1.15, 2.32; P = 0.0058) (Fig. 4).
Discussion
In this study, we compared the efficacy and safety of the long-acting GnRH agonist follicular protocol with the GnRH antagonist protocols among patients undergoing ART. With regards to effectiveness, the main outcome of our study (LBR) and secondary outcomes (clinical pregnancy rate and the implantation rate) were higher in the long-acting GnRH agonist follicular protocol compared with GnRH antagonist protocol, and this association was found to be statistically significant. Regarding safety, the incidence of OHSS was lower in the GnRH antagonist protocol compared to the long-acting GnRH agonist follicular protocol.
Long-acting GnRH agonist protocols, which enable maximum ovarian stimulation, have been the standard IVF protocol since decades [24]. The long-acting GnRH agonist protocol has advantages over the GnRH antagonist, primarily by complete elimination of the fluctuation in preovulatory LH levels during the course of ovarian hyperstimulation [1]. A decreased probability of pregnancy due to the increased incidence of LH instability in the GnRH antagonist cycles has been evaluated by many studies [25,26,27]. In our study, the potential benefits of the long-acting GnRH agonist follicular protocol with regard to live birth rate, clinical pregnancy rate, and implantation rate were observed, especially for normal ovarian responders, as our study had 69.2% of normal ovarian responders. In addition, the antagonist protocol was more likely to be suitable for patients with PCOS with regard to lower OHSS rate and higher proportion of this type of patient involved (46.6%).
The fact that in the literature the GnRH antagonist protocol demonstrated a similar pregnancy outcome could be explained by several factors. Firstly, a greater number of studies used the GnRH antagonist protocol owing to relatively less complexity and desirable outcomes offered by the antagonist protocol, which includes mild ovarian stimulation, patient-compatible regimen, and lower risk of OHSS [25]. Secondly, there could be publication bias in the inclusion of larger studies. As a fact, long-acting GnRH agonist follicular protocols are extensively used in China and studies published in Chinese are excluded from the majority of meta-analyses published internationally [8].
A recent systematic review and meta-analysis by Lambalk et al. [8] compared ovarian stimulation protocols involving various patients, such as couples undergoing IVF in the general population, women with PCOS and poor ovarian response. Our meta-analysis revealed that, in the general IVF population, the long-acting agonist protocol remains the superior treatment of choice by resulting in better ongoing pregnancy rate compared with antagonist protocol. However, among PCOS and poor ovarian responders, the GnRH antagonist protocol seems to be the standard choice of treatment as it is associated with a lesser rate of OHSS [8]. Other studies have shown no difference in live birth rate between the long-acting GnRH agonist and antagonist protocols [23, 25,26,27,28]. However, a study conducted by Lambalk et al. [8] suggested that ongoing pregnancy can be considered as a good proxy for live birth rate, although a discrepancy exists between the live birth rate and ongoing pregnancy rate, and reporting of ongoing pregnancy rate is sufficiently powered to detect the ideal differences of the effectiveness of treatments [29].
In our study, compared with the GnRH antagonist protocol, the long-acting GnRH agonist follicular protocol resulted in higher clinical pregnancy rate and implantation rate. Similarly, a Cochrane review conducted by Al-Inany et al. [30] showed results in favor of the long-acting GnRH agonist protocol. In contrast, no statistically significant differences in clinical pregnancy rate between both the protocols were observed in other studies [31]. This difference in results could plausibly be attributed to the number of studies and patients included in these analyses, as well as the inclusion of studies using the long luteal protocol and not the long agonist follicular protocol.
It is well documented that administration of exogeneous GnRH agonists or GnRH antagonist for ovarian stimulation in IVF can lead to OHSS [1]. A substantial amount of evidence suggests that the GnRH antagonist protocol decreases the risk of OHSS in patients undergoing [21]. Likewise, in our study, the GnRH antagonist protocol has shown lower rates of OHSS. A Cochrane systematic review also reported similar findings [32]. In our meta-analysis women undergoing IVF with the GnRH antagonist protocol showed lower incidence of OHSS compared to those who received the long-acting GnRH agonist follicular protocol. Additionally, our results showed that the follicular long-acting protocol is more widely used in China than in Western population. Hence, our findings highlight the advantages of the follicular long-acting protocol over the antagonist protocol in IVF.
Strength and Limitations of the Study
To the best of our knowledge, this is the first meta-analysis comparing the long-acting GnRH agonist follicular and GnRH antagonist protocols by undertaking a comprehensive literature search that includes English-language and Chinese articles. However, our study has few limitations. First, a limited number of studies published in English were included, which could lead to bias as the results cannot be generalized to the wider population. Second, a limited number of studies assessing live birth rate could also create bias in the analysis and interpretation of the results. Third, owing to the limited number of studies, non-RCTs, retrospective studies, studies with small sample size and various study populations with variation in ovarian responses were included in the analysis.
In conclusion, our results revealed significantly higher live birth, clinical pregnancy, and implantation rates with the GnRH agonist protocol than with the GnRH antagonists protocol. With regard to safety, especially for hyperresponsive patients, the GnRH antagonist protocol substantially reduced the risk of OHSS.
References
Allahbadia GN. The ideal stimulation protocol: is there one? J Obstet Gynaecol India. 2015;65:357–61.
van den Wijngaard L, van Wely M, Dancet EAF, et al. Patients’ preferences for gonadotrophin-releasing hormone analogs in in vitro fertilization. Gynecol Obstet Investig. 2014;78:16–21.
Hughes EG, Fedorkow DM, Daya S, Sagle MA, Van de Koppel P, Collins JA. The routine use of gonadotropin-releasing hormone agonists prior to in vitro fertilization and gamete intrafallopian transfer: a meta-analysis of randomized controlled trials. Fertil Steril. 1992;58:888–96.
Shalev E, Leung PCK. Gonadotropin-releasing hormone and reproductive medicine. J Obstet Gynaecol Can. 2003;25:98–113.
Geng Y, Xun Y, Hu S, Lai Q, Jin L. GnRH antagonist versus follicular-phase single-dose GnRH agonist protocol in patients of normal ovarian responses during controlled ovarian stimulation. Gynecol Endocrinol. 2019;35:309–13.
Surrey ES, Silverberg KM, Surrey MW, Schoolcraft WB. Effect of prolonged gonadotropin-releasing hormone agonist therapy on the outcome of in vitro fertilization-embryo transfer in patients with endometriosis. Fertil Steril. 2002;78:699–704.
Ren J, Sha A, Han D, Li P, Geng J, Ma C. Does prolonged pituitary down-regulation with gonadotropin-releasing hormone agonist improve the live-birth rate in in vitro fertilization treatment? Fertil Steril. 2014;102:75–81.
Lambalk CB, Banga FR, Huirne JA, et al. GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Hum Reprod Update. 2017;23:560–79.
Albuquerque LET, Tso LO, Saconato H, Albuquerque MCRM, Macedo CR. Depot versus daily administration of gonadotrophin-releasing hormone agonist protocols for pituitary down regulation in assisted reproduction cycles. Cochrane Database Syst Rev. 2013;CD002808.
Wang R, Lin S, Wang Y, Qian W, Zhou L. Comparisons of GnRH antagonist protocol versus GnRH agonist long protocol in patients with normal ovarian reserve: a systematic review and meta-analysis. PLoS One. 2017;12(4):e0175985.
Gordts S, Van Turnhout C, Campo R, Puttemans P, Valkenburg M, Gordts S. A prospective randomised study comparing a GnRH-antagonist versus a GnRH-agonist short protocol for ovarian stimulation in patients referred for IVF. Facts Views Vis ObGyn. 2012;4:82–7.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Clark HD, Wells GA, Huët C, et al. Assessing the quality of randomized trials: reliability of the Jadad scale. Control Clin Trials. 1999;20:448–52.
Yang R, Luo L, Wang Y, et al. Clinical protocol selection in patients with polycystic ovary syndrome who undergone the first IVF/ICSI-ET treatment. Reprod Contracept. 2015;35:241–6.
Hu YQ, Cai JL, Liu LL, et al. Clinical outcomes of modified long protocol used in patients who failed in first cycle with antagonist protocol. Prog Obstet Gynecol. 2014;23:455–8.
Zhang Y, Bao JH, Yao HR, et al. Clinical application and economic analysis of gonadotropin-releasing hormone antagonist protocol in patients with decreased ovarian reserve. Reprod Contracept. 2018;38:228–31.
Liu N, Ma Y, Li R, et al. Comparison of follicular fluid amphiregulin and EGF concentrations in patients undergoing IVF with different stimulation protocols. Endocrine. 2012;42:708–16.
Zhao J. Clinical application analysis of long acting follicular-phase GnRH agonist versus GnRH antagonist protocol in patients with polycystic ovary syndrome undergoing controlled ovarian stimulation. Chin J Woman Child Health Res. 2017;28:430–1.
Xu DF, Wu QF. Application and effect of super long project and GnRH-antagnist in IVF-ET with the patients of PCOS. Jiangxi Med J. 2015;50:13–5.
Xu HL, Zheng BH, Qiu SM, et al. Selection of controlled ovarian hyperstimualtion in patients with polycystic ovary syndrome who undergone in vitro fertilization. Strait J Prev Med. 2017;23:94–6.
Liu L, Zhao YQ, Liu P, et al. The clinical analysis of GnRH antagonist protocols on PCOS patients. Ningxia Med J. 2015;37:818–20.
Tian LF, Wu QF, Su Q, et al. A pregnancy outcomes comparison of low ovarian response in infertile patients undergoing different controlled ovarian hyperstimulation protocols in IVF treatment. Jianxi Med J. 2013;48:479–82.
Zhao ZM, Hao GM, Cui N, et al. Impact of long protocol in early follicular phase of patients with polycystic ovary syndrome who undergone in vitro fertilizaton-embryo transfer on clinical outcomes. Chin J Fam Plan. 2018;26:709–13.
Daya S. Gonadotropin releasing hormone agonist protocols for pituitary desensitization in in vitro fertilization and gamete intrafallopian transfer cycles. Cochrane Database Syst Rev. 2000;(2):CD001299.
Kolibianakis EM, Albano C, Camus M, Tournaye H, Van Steirteghem AC, Devroey P. Initiation of gonadotropin-releasing hormone antagonist on day 1 as compared to day 6 of stimulation: effect on hormonal levels and follicular development in in vitro fertilization cycles. J Clin Endocrinol Metab. 2003;88:5632–7.
Seow K-M, Lin Y-H, Hsieh B-C, et al. Characteristics of progesterone changes in women with subtle progesterone rise in recombinant follicle-stimulating hormone and gonadotropin-releasing hormone antagonist cycle. Gynecol Obstet Investig. 2010;70:64–8.
Bosch E, Valencia I, Escudero E, et al. Premature luteinization during gonadotropin-releasing hormone antagonist cycles and its relationship with in vitro fertilization outcome. Fertil Steril. 2003;80:1444–9.
Al-Inany HG, Youssef MA, Aboulghar M, et al. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev. 2011;CD001750.
Braakhekke M, Kamphuis EI, Dancet EA, Mol F, van der Veen F, Mol BW. Ongoing pregnancy qualifies best as the primary outcome measure of choice in trials in reproductive medicine: an opinion paper. Fertil Steril. 2014;101:1203–4.
Al-Inany HG, Abou-Setta AM, Aboulghar M. Gonadotrophin-releasing hormone antagonists for assisted conception: a Cochrane review. Reprod Biomed Online. 2007;14:640–9.
Xiao J, Su C, Zeng X. Comparisons of GnRH antagonist versus GnRH agonist protocol in supposed normal ovarian responders undergoing IVF: a systematic review and meta-analysis. PLoS One. 2014;9:e106854.
Al-Inany HG, Youssef MA, Ayeleke RO, Brown J, Lam WS, Broekmans FJ. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev. 2016;4:CD001750.
Acknowledgements
Funding
The study was funded by Ipsen and also Ipsen funded the journal’s Rapid Service Fee. Ipsen funded the Open Access Fee.
Medical Writing and Editorial Assistance
The authors thank Dr. Anuradha Nalli and Pingping Wang of Indegene Pvt. Ltd, for providing medical writing support, article screening and statistical analysis which was sponsored by Ipsen China in accordance with Good Publication Practice guidelines.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Rui Yang, Yichun Guan and Rong Li have nothing to disclose. Valerie Perrot and Juan Ma are employees of Ipsen.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yang, R., Guan, Y., Perrot, V. et al. Comparison of the Long-Acting GnRH Agonist Follicular Protocol with the GnRH Antagonist Protocol in Women Undergoing In Vitro Fertilization: A Systematic Review and Meta-analysis. Adv Ther 38, 2027–2037 (2021). https://doi.org/10.1007/s12325-020-01612-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01612-7