Abstract
Purpose
The effect of PTEN inhibitor (Bpv(HOpic); Bpv) and mTOR activators (phosphatidic acid (PA) and propranolol (PP)), were evaluated on the activation and subsequent development of human primordial follicles in ovarian tissue culture.
Methods
Slow frozen-thawed human ovarian cortical strips were incubated for 24 h in different groups: (1) Control (base medium), (2) Bpv (100 µM), (3) PA (200 µM), (4) PA + PP (50 µm), and (5) Bpv + PA + PP. Afterward, the medium was exchanged, and all groups were cultured without stimulators for 6 additional days. The proportion of normal and degenerated follicles, estradiol secretion, and expression of RPS6, FOXO3a, and AKT proteins was evaluated and compared between groups.
Results
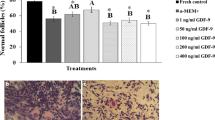
After 24 h of culture, there was no significant difference between the proportion of primordial and growing follicles in either of the experimental groups. This non-significant change was also observed for the phosphorylated protein to total protein ratios of RPS6, FOXO3a, and AKT proteins. After 7 days of culture, the proportion of the transitional follicles was significantly higher in comparison to the primordial follicles for the PA, PA + PP, and Bpv + PA + PP groups. The estradiol level was significantly higher on the last day compared to the first day, in PA, PA + PP, and Bpv + PA + PP groups. Hormonal secretion was significantly higher in the PA and PA + PP groups and lower in the Bpv and Bpv + PA + PP groups compared to the control on day 7 of culture.
Conclusion
Temporary in vitro treatment of human ovarian tissue with mTOR activators enhances the initiation of primordial follicle development and positively influences steroidogenesis after short-term culture.




Similar content being viewed by others
Data availability and material
Not applicable.
Code availability
Not applicable.
References
Dolmans M-M, Cordier F, A Amorim C, Donnez J, Vander Linden CJFie In vitro activation prior to transplantation of human ovarian tissue: is it truly effective? 2019; 10:520
Ernst EH, Grøndahl ML, Grund S, Hardy K, Heuck A, Sunde L et al. Dormancy and activation of human oocytes from primordial and primary follicles: molecular clues to oocyte regulation. 2017;32(8):1684-1700
John GB, Shirley LJ, Gallardo TD. Castrillon DH Specificity of the requirement for Foxo3 in primordial follicle activation. Reproduction (Cambridge, England). 2007;133(5):855–63.
Tarnawa ED, Baker MD, Aloisio GM, Carr BR, Castrillon DH Gonadal expression of Foxo1, but not Foxo3, is conserved in diverse Mammalian species. Biol Reprod. 2013;88(4):103, 101–111
Albamonte MI, Calabró LY, Albamonte MS, Zuccardi L, Stella I, Halperin J et al. PTEN and FOXO3 expression in the prenatal and postnatal human ovary. J Assisted Reprod Genet. 2020:1–10
Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science (New York, NY). 2008;319(5863):611–3.
Grosbois J, Demeestere IJHr Dynamics of PI3K and Hippo signaling pathways during in vitro human follicle activation. 2018;33(9):1705–1714
Sato Y, Kawamura KJM, Endocrinology C rapamycin treatment maintains developmental potential of oocytes in mice and follicle reserve in human cortical fragments grafted into immune-deficient mice. 2020; 504:110694
Masciangelo R, Hossay C, Chiti MC, Manavella DD, Amorim CA, Donnez J, et al. Role of the PI3K and Hippo pathways in follicle activation after grafting of human ovarian tissue. J Assisted Reprod. 2020;37(1):101–8.
Li J, Kawamura K, Cheng Y, Liu S, Klein C, Liu S, et al. Activation of dormant ovarian follicles to generate mature eggs. 2010;107(22):10280–4.
Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci. 2013;110(43):17474–9.
Sun X, Su Y, He Y, Zhang J, Liu W, Zhang H et al. New strategy for in vitro activation of primordial follicles with mTOR and PI3K stimulators. 2015;14(5):721–731
Lerer-Serfaty G, Samara N, Fisch B, Shachar M, Kossover O, Seliktar D, et al. Attempted application of bioengineered/biosynthetic supporting matrices with phosphatidylinositol-trisphosphate-enhancing substances to organ culture of human primordial follicles. J Assist Reprod Genet. 2013;30(10):1279–88. https://doi.org/10.1007/s10815-013-0052-8.
McLaughlin M, Kinnell HL, Anderson RA, Telfer EEJMhr Inhibition of phosphatase and tensin homologue (PTEN) in human ovary in vitro results in increased activation of primordial follicles but compromises development of growing follicles. 2014;20(8):736–744
Novella-Maestre E, Herraiz S, Rodríguez-Iglesias B, Díaz-García C, Pellicer AJPO Short-term PTEN inhibition improves in vitro activation of primordial follicles, preserves follicular viability, and restores AMH levels in cryopreserved ovarian tissue from cancer patients. 2015;10(5)
Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, et al. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. 2015;30(3):608–15.
Zhai J, Yao G, Dong F, Bu Z, Cheng Y, Sato Y, et al. In vitro activation of follicles and fresh tissue auto-transplantation in primary ovarian insufficiency patients. 2016;101(11):4405–12.
Ghezelayagh Z, Abtahi NS, Khodaverdi S, Valojerdi MR, Mehdizadeh A. Ebrahimi B The effect of agar substrate on growth and development of cryopreserved-thawed human ovarian cortical follicles in organ culture. Eur J Obstet Gynecol Reprod Biol. 2021;258:139–45.
Dalman A, Farahani NSDG, Totonchi M, Pirjani R, Ebrahimi B, Valojerdi MRJC Slow freezing versus vitrification technique for human ovarian tissue cryopreservation: an evaluation of histological changes, WNT signaling pathway and apoptotic genes expression. 2017;79:29-36
Ghezelayagh Z, Abtahi NS, Valojerdi MR, Mehdizadeh A. Ebrahimi B The combination of basic fibroblast growth factor and kit ligand promotes the proliferation, activity and steroidogenesis of granulosa cells during human ovarian cortical culture. Cryobiology. 2020;96:30–6.
Blanco-Aparicio C, Renner O, Leal JF, Carnero AJC PTEN, more than the AKT pathway. 2007;28(7):1379-1386
Garor R, Abir R, Erman A, Felz C, Nitke S. Fisch B Effects of basic fibroblast growth factor on in vitro development of human ovarian primordial follicles. Fertil Steril. 2009;91(5 Suppl):1967–75. https://doi.org/10.1016/j.fertnstert.2008.04.075.
Kedem A, Fisch B, Garor R, Ben-Zaken A, Gizunterman T, Felz C, et al. Growth differentiating factor 9 (GDF9) and bone morphogenetic protein 15 both activate development of human primordial follicles in vitro, with seemingly more beneficial effects of GDF9. J Clin Endocrinol Metab. 2011;96(8):E1246-1254. https://doi.org/10.1210/jc.2011-0410.
Funding
This research was financially supported by the Royan Institute.
Author information
Authors and Affiliations
Contributions
Zeinab Ghezelayagh: PhD student, investigation, methodology, formal analysis, writing—original draft.
Naeimeh Sadat Abtahi: Troubleshooting in the lab, data curation, follicular count, writing—review and editing.
Mojtaba Rezazadeh Valojerdi: Scientific adviser of the project, writing—review and editing.
Bita Ebrahimi: Supervision, conceptualization, project administration, writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Ethics Committee of Royan Institute (ethics code: IR.ACECR.ROYAN.REC.1396.212).
Consent to participate
Informed consent was received from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key messages
In vitro activation of primordial follicles via various pharmacological compounds provides an alternative technique for fertility restoration. Here we indicate that in vitro treatment of human ovarian tissue with mTOR activators enhances the initiation of primordial follicle development and positively influences steroidogenesis.
Rights and permissions
About this article
Cite this article
Ghezelayagh, Z., Abtahi, N.S., Rezazadeh Valojerdi, M. et al. The effect of mTOR activation and PTEN inhibition on human primordial follicle activation in ovarian tissue culture. J Assist Reprod Genet 39, 1739–1747 (2022). https://doi.org/10.1007/s10815-022-02537-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02537-6