Abstract
Purpose
To determine whether blastocyst morphology has an impact on sex proportion at pre-implantation and birth in PGT-A and non-PGT-A cycles.
Methods
A total of 1254 biopsied blastocysts from 466 PGT-A cycles were analyzed for sex proportion, day of biopsy, degree of expansion, inner cell mass (ICM), and trophectoderm (TE) morphology. From these, 197 frozen single embryo transfers (SET) were assessed for clinical outcomes and sex proportion of ongoing pregnancies and deliveries. In addition, we evaluated the day of vitrification/embryo transfer, degree of expansion, and TE morphology in a group of 229 births (217 cycles) from frozen or fresh transfers of non-biopsied blastocysts.
Results
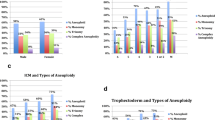
Sex proportion was impacted by day of biopsy and TE morphology, but not by ICM morphology, in PGT-A cycles. Therefore, biopsy on day 5 and TE “A” shifted the sex proportion towards males. Interestingly, we noted that our morphology-based embryo selection for SET of euploid blastocysts has favored the choice for XY embryos, generating a 54.3% XY proportion at transfer and 56.1% XY proportion at ongoing pregnancy/delivery. Our models indicate a weaker association between blastocyst morphology parameters and sex proportion of babies in non-PGT-A cycles.
Conclusion
Blastocyst features associated with a skewed sex proportion towards XY embryos, such as biopsy on day 5 and top quality TE, are also parameters used for selecting euploid embryos for SET. Therefore, our data suggest that morphology-based embryo selection represents a strong factor responsible for a skewed male sex proportion at birth in PGT-A cycles.

Similar content being viewed by others
Data availability
Not applicable
References
Hesketh T, Xing ZW. Abnormal sex ratios in human populations: causes and consequences. Proc Natl Acad Sci U S A. 2006;103:13271–5.
Austad SN. The human prenatal sex ratio a major surprise. Proc Natl Acad Sci U S A. 2015;112:4839–40.
Sunderam S, Kissin DM, Zhang Y, Jewett A, Boulet SL, Warner L, et al. Assisted reproductive technology surveillance — United States, 2017. MMWR Surveill Summ. 2020;69:1–24.
Gliozheni O, Hambartsoumian E, Strohmer H, Petrovskaya E, Tishkevich O, Bogaerts K, et al. ART in Europe, 2016: results generated from European registries by ESHRE†. Hum Reprod Open. 2020;2020:1–17.
De Croo I, Colman R, De Sutter P, Tilleman K. Blastocyst transfer for all? Higher cumulative live birth chance in a blastocyst-stage transfer policy compared to a cleavage-stage transfer policy. Facts, views Vis ObGyn. 2019;11:169–76.
Ménézo YJ, Chouteau J, Torelló M, Girard A, Veiga A. Birth weight and sex ratio after transfer at the blastocyst stage in humans. Fertil Steril. 1999;72:221–4.
Milki AA, Jun SH, Hinckley MD, Westphal LW, Giudice LC, Behr B. Comparison of the sex ratio with blastocyst transfer and cleavage stage transfer. J Assist Reprod Genet. 2003;20:323–6.
Maalouf WE, Mincheva MN, Campbell BK, Hardy ICW. Effects of assisted reproductive technologies on human sex ratio at birth. Fertil Steril. Elsevier Inc. 2014;101:1321–5. https://doi.org/10.1016/j.fertnstert.2014.01.041.
Dean JH, Chapman MG, Sullivan EA. The effect on human sex ratio at birth by assisted reproductive technology (ART) procedures - an assessment of babies born following single embryo transfers, Australia and New Zealand, 2002-2006. BJOG An Int J Obstet Gynaecol. 2010;117:1628–34.
Chen M, Du J, Zhao J, Lv H, Wang Y, Chen X, et al. The sex ratio of singleton and twin delivery offspring in assisted reproductive technology in China. Sci Rep. Springer US. 2017;7:1–8. https://doi.org/10.1038/s41598-017-06152-9.
Luna M, Duke M, Copperman A, Grunfeld L, Sandler B, Barritt J. Blastocyst embryo transfer is associated with a sex-ratio imbalance in favor of male offspring. Fertil Steril. Elsevier. 2007;87:519–23.
Shaia K, Truong T, Pieper C, Steiner A. Pre-implantation genetic testing alters the sex ratio: an analysis of 91,805 embryo transfer cycles. J Assist Reprod Genet. 2020;37:1117–22.
Avery B, Madison V, Greve T. Sex and development in bovine in-vitro fertilized embryos. Theriogenology. 1991;35:953–63.
Valdivia RPA, Kunieda T, Azuma S, Toyoda Y. PCR sexing and developmental rate differences in preimplantation mouse embryos fertilized and cultured in vitro. Mol Reprod Dev. 1993;35:121–6.
Bernardi ML, Delouis C. Sex-related differences in the developmental rate of in-vitro matured/in-vitro fertilized ovine embryos. Hum Reprod. 1996;11:621–6.
Cassar G, King WA, King GJ. Influence of sex on early growth of pig conceptuses. Reproduction. 1994;101:317–20.
Pegoraro LM, Thuard J, Delalleau N, Guérin B, Deschamps J, Marquant-Le Guienne B, et al. Comparison of sex ratio and cell number of ivm-ivf bovine blastocysts co-cultured with bovine oviduct epithelial cells or with vero cells. Theriogenology. 1998;49:1579–90.
Sidrat T, Kong R, Khan AA, Idrees M, Xu L, El Sheikh M, et al. Difference in developmental kinetics of Y-specific monoclonal antibody sorted male and female in vitro produced bovine embryos. Int J Mol Sci. 2020;21.
Kawase Y, Tachibe T, Kamada N, Ichi JK, Watanabe H, Suzuki H. Male advantage observed for in vitro fertilization mouse embryos exhibiting early cleavage. Reprod Med Biol. 2021;20:83–7.
Ray PF, Conaghan J, Winston RML, Handyside AH. Increased number of cells and metabolic activity in male human preimplantation embryos following in vitro fertilization. Reproduction. 1995;104:165–71.
Alfarawati S, Fragouli E, Colls P, Stevens J, Gutiérrez-Mateo C, Schoolcraft WB, et al. The relationship between blastocyst morphology, chromosomal abnormality, and embryo gender. Fertil Steril. Elsevier. 2011;95:520–4.
Bronet F, Nogales MC, Martínez E, Ariza M, Rubio C, García-Velasco JA, et al. Is there a relationship between time-lapse parameters and embryo sex? Fertil Steril. Elsevier Inc. 2015;103:396–401.e2. https://doi.org/10.1016/j.fertnstert.2014.10.050.
Huang B, Ren X, Zhu L, Wu L, Tan H, Guo N, et al. Is differences in embryo morphokinetic development significantly associated with human embryo sex? Biol Reprod. 2019;100:618–23.
Ebner T, Tritscher K, Mayer RB, Oppelt P, Duba HC, Maurer M, et al. Quantitative and qualitative trophectoderm grading allows for prediction of live birth and gender. J Assist Reprod Genet. 2016;33:49–57.
Bermejo-Alvarez P, Rizos D, Lonergan P, Gutierrez-Adan A. Transcriptional sexual dimorphism during preimplantation embryo development and its consequences for developmental competence and adult health and disease. Reproduction. 2011;141:563–70.
Zorrilla M, Yatsenko AN. The genetics of infertility: current status of the field. Curr Genet Med Rep. Springer. 2013;1:247–60.
Gelbaya TA, Potdar N, Jeve YB, Nardo LG. Definition and epidemiology of unexplained infertility. Obstet Gynecol Surv. LWW. 2014;69:109–15.
Roos Kulmann MI, Lumertz Martello C, Bos-Mikich A, Frantz N. Pronuclear and blastocyst morphology are associated age-dependently with embryo ploidy in in vitro fertilization cycles. Hum Fertil. Taylor & Francis. 2020:1–8.
Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. Elsevier. 2000;73:1155–8.
Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, et al. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod. 2014;29:1173–81.
Rubio C, Rodrigo L, Garcia-Pascual C, Peinado V, Campos-Galindo I, Garcia-Herrero S, et al. Clinical application of embryo aneuploidy testing by next-generation sequencing. Biol Reprod. Oxford University Press. 2019;101:1083–90.
Wang A, Kort J, Behr B, Westphal L. Euploidy in relation to blastocyst sex and morphology. J Assist Reprod Genet. 2018;35:1565–72.
Gardner DK, Wale PL, Collins R, Lane M. Glucose consumption of single post-compaction human embryos is predictive of embryo sex and live birth outcome. Hum Reprod. 2011;26:1981–6.
Gardner DK, Larman MG, Thouas GA. Sex-related physiology of the preimplantation embryo. Mol Hum Reprod. 2010;16:539–47.
Sturmey RG, Bermejo-Alvarez P, Gutierrez-Adan A, Rizos D, Leese HJ, Lonergan P. Amino acid metabolism of bovine blastocysts: a biomarker of sex and viability. Mol Reprod Dev. 2010;77:285–96.
Petropoulos S, Edsgärd D, Reinius B, Deng Q, Panula SP, Codeluppi S, et al. Single-cell RNA-Seq reveals lineage and X chromosome dynamics in human preimplantation embryos. Cell. 2016;165:1012–26.
De Mello JCM, Fernandes GR, Vibranovski MD, Pereira LV. Early X chromosome inactivation during human preimplantation development revealed by single-cell RNA-sequencing. Sci Rep. Springer US. 2017;7:1–12. https://doi.org/10.1038/s41598-017-11044-z.
Code availability
Not applicable
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Consent to participate
All patients had previously given informed consent for the use of their data.
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Roos Kulmann, M.I., Lumertz Martello, C., Mezzomo Donatti, L. et al. Morphology-based selection from available euploid blastocysts induces male-skewed sex proportion in the offspring. J Assist Reprod Genet 38, 2165–2172 (2021). https://doi.org/10.1007/s10815-021-02235-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-021-02235-9