Abstract
Purpose
To study the use of in silica model to better understand and propose new markers of ovarian response to controlled ovarian stimulation before IVF.
Methods
A systematic review and in silica model using bioinformatics. After the selection of 103 papers from a systematic review process, we performed a GRADE qualification of all included papers for evidence-based quality evaluation. We included 57 genes in the silica model using a functional protein network interaction. Moreover, the construction of protein-protein interaction network was done importing these results to Cytoscape. Therefore, a cluster analysis using MCODE was done, which was exported to a plugin BINGO to determine Gene Ontology. A p value of < 0.05 was considered significant, using a Bonferroni correction test.
Results
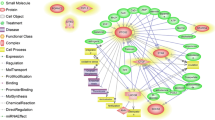
In silica model was robust, presenting an ovulation-related gene network with 87 nodes (genes) and 348 edges (interactions between the genes). Related to the network centralities, the network has a betweenness mean value = 102.54; closeness mean = 0.007; and degree mean = 8.0. Moreover, the gene with a higher betweenness was PTPN1. Genes with the higher closeness were SRD5A1 and HSD17B3, and the gene with the lowest closeness was GDF9. Finally, the gene with a higher degree value was UBB; this gene participates in the regulation of TP53 activity pathway.
Conclusions
This systematic review demonstrated that we cannot use any genetic marker before controlled ovarian stimulation for IVF. Moreover, in silica model is a useful tool for understanding and finding new markers for an IVF individualization.
Prospero
CRD42020197185






Similar content being viewed by others
Data availability
All data are available upon request.
References
Altmäe S, Hovatta O, Stavreus-Evers A, Salumets A. Genetic predictors of controlled ovarian hyperstimulation: where do we stand today? Hum Reprod Update. 2011;17:813–28.
Kligman I, Rosenwaks Z. Differentiating clinical profiles: predicting good responders, poor responders, and hyperresponders. Fertil Steril. 2001;76:1185–90.
Coccia ME, Rizzello F. Ovarian reserve. Ann N Y Acad Sci. 2008;1127:27–30.
Olivennes F, Howies CM, Borini A, Germond M, Trew G, Wikland M, et al. Individualizing FSH dose for assisted reproduction using a novel algorithm: the CONSORT study. Reprod BioMed Online. 2011;22(Suppl 1):S73–82.
Keck C, Bassett R, Ludwig M. Factors influencing response to ovarian stimulation. Reprod BioMed Online. 2005;11:562–9.
Alviggi C, Humaidan P, Ezcurra D. Hormonal, functional and genetic biomarkers in controlled ovarian stimulation: tools for matching patients and protocols. Reprod Biol Endocrinol. 2012;10:9.
Broekmans FJ, Kwee J, Hendriks DJ, Mol BW. Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12:685–718.
Zheng H, Chen S, Du H, Ling J, Wu Y, Liu H, et al. Ovarian response prediction in controlled ovarian stimulation for IVF using anti-Müllerian hormone in Chinese women: a retrospective cohort study. Medicine. 2017;96:e6495.
Li Y, Nie M, Liu Y, Zhang W, Yang X. The dynamic changes of anti-Mullerian hormone and inhibin B during controlled ovarian hyperstimulation in decreased ovarian reserve women and the effect on clinical outcome. Gynecol Endocrinol. 2015;31:450–3.
De Conto E, Genro VK, da Silva DS, Chapon R de CB, Cunha-Filho JSL. AMH as a prognostic factor for blastocyst development. JBRA Assist Reprod. 2015;19:131–4.
Bessow C, Donato R, de Souza T, Chapon R, Genro V, Cunha-Filho JS. Antral follicle responsiveness assessed by follicular output RaTe(FORT) correlates with follicles diameter. J Ovarian Res. 2019;12:48.
Genro VK, Matte U, De Conto E, Cunha-Filho JS, Fanchin R. Frequent polymorphisms of FSH receptor do not influence antral follicle responsiveness to follicle-stimulating hormone administration as assessed by the Follicular Output RaTe (FORT). J Assist Reprod Genet. 2012;29:657–63.
Gallot V, Berwanger da Silva AL, Genro V, Grynberg M, Frydman N, Fanchin R. Antral follicle responsiveness to follicle-stimulating hormone administration assessed by the Follicular Output RaTe (FORT) may predict in vitro fertilization-embryo transfer outcome. Hum Reprod. 2012;27:1066–72.
Rehman R, Mustafa R, Baig M, Arif S, Hashmi MF. Use of Follicular Output Rate to predict intracytoplasmic sperm injection outcome. Int J Fertil Steril. 2016;10:169–74.
de Castro F, Morón FJ, Montoro L, Real LM, Ruiz A. Pharmacogenetics of controlled ovarian hyperstimulation. Pharmacogenomics. 2005;6:629–37.
García-Jiménez G, Zariñán T, Rodríguez-Valentín R, Mejía-Domínguez NR, Gutiérrez-Sagal R, Hernández-Montes G, et al. Frequency of the T307A, N680S, and -29G>A single-nucleotide polymorphisms in the follicle-stimulating hormone receptor in Mexican subjects of Hispanic ancestry. Reprod Biol Endocrinol. 2018;16:100.
Klinkert ER, te Velde ER, Weima S, van Zandvoort PM, Hanssen RG, Nilsson PR, et al. FSH receptor genotype is associated with pregnancy but not with ovarian response in IVF. Reprod BioMed Online. 2006:687–95.
Karagiorga I, Partsinevelos GA, Mavrogianni D, Anagnostou E, Zervomanolakis I, Kallianidis K, et al. Single nucleotide polymorphisms in the anti-Müllerian hormone (AMH Ile(49)Ser) and anti-Müllerian hormone type II receptor (AMHRII -482 A>G) as genetic markers in assisted reproduction technology. J Assist Reprod Genet. 2015;32:357–67.
Oliveira JBA, Baruffi RLR, Petersen CG, Mauri AL, Nascimento AM, Vagnini L, et al. A new ovarian response prediction index (ORPI): implications for individualised controlled ovarian stimulation. Reprod Biol Endocrinol. 2012;10:94.
Chalumeau C, Moreau J, Gatimel N, Cohade C, Lesourd F, Parinaud J, et al. Establishment and validation of a score to predict ovarian response to stimulation in IVF. Reprod BioMed Online. 2018;36:26–31.
Colquitt RB, Colquhoun DA, Thiele RH. In silico modelling of physiologic systems. Best Pract Res Clin Anaesthesiol. 2011;25:499–510.
Jaeschke R, Guyatt GH, Dellinger P, Schünemann H, Levy MM, Kunz R, et al. Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ. 2008;a744:337.
Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–13.
Pathan M, Keerthikumar S, Ang C-S, Gangoda L, Quek CYJ, Williamson NA, et al. FunRich: an open access standalone functional enrichment and interaction network analysis tool. Proteomics. 2015;15:2597–601.
Achrekar SK, Modi DN, Desai SK, Mangoli VS, Mangoli RV, Mahale SD. Poor ovarian response to gonadotrophin stimulation is associated with FSH receptor polymorphism. Reprod BioMed Online. 2009;18:509–15.
Aflatoonian A, Oskouian H, Ahmadi S, Oskouian L. Prediction of high ovarian response to controlled ovarian hyperstimulation: anti-Müllerian hormone versus small antral follicle count (2-6 mm). J Assist Reprod Genet. 2009;26:319–25.
Alson SSE, Bungum LJ, Giwercman A, Henic E. Anti-müllerian hormone levels are associated with live birth rates in ART, but the predictive ability of anti-müllerian hormone is modest. Eur J Obstet Gynecol Reprod Biol. 2018;225:199–204.
Alviggi C, Pettersson K, Longobardi S, Andersen CY, Conforti A, De Rosa P, et al. A common polymorphic allele of the LH beta-subunit gene is associated with higher exogenous FSH consumption during controlled ovarian stimulation for assisted reproductive technology. Reprod Biol Endocrinol. 2013;11:51.
Anckaert E, Smitz J, Schiettecatte J, Klein BM, Arce CJ. The value of anti-Mullerian hormone measurement in the long GnRH agonist protocol: association with ovarian response and gonadotrophin-dose adjustments. Hum Reprod. 2012;27:1829–39.
Andersen AN, Witjes H, Gordon K, Mannaerts B. Xpect investigators. Predictive factors of ovarian response and clinical outcome after IVF/ICSI following a rFSH/GnRH antagonist protocol with or without oral contraceptive pre-treatment. Hum Reprod. 2011;26:3413–23.
Arce J-C, La Marca A, Mirner Klein B, Nyboe Andersen A, Fleming R. Antimüllerian hormone in gonadotropin releasing-hormone antagonist cycles: prediction of ovarian response and cumulative treatment outcome in good-prognosis patients. Fertil Steril. 2013;99:1644–53.
Ashrafi M, Madani T, Tehranian AS, Malekzadeh F. Follicle stimulating hormone as a predictor of ovarian response in women undergoing controlled ovarian hyperstimulation for IVF. Int J Gynaecol Obstet. 2005;91:53–7.
Bahceci M, Ulug U, Turan E, Akman MA. Comparisons of follicular levels of sex steroids, gonadotropins and insulin like growth factor-1 (IGF-1) and epidermal growth factor (EGF) in poor responder and normoresponder patients undergoing ovarian stimulation with GnRH antagonist. Eur J Obstet Gynecol Reprod Biol. 2007;130:93–8.
Baker VL, Gracia C, Glassner MJ, Schnell VL, Doody K, Coddington CC, et al. Multicenter evaluation of the Access AMH antimüllerian hormone assay for the prediction of antral follicle count and poor ovarian response to controlled ovarian stimulation. Fertil Steril. 2018;110:506–13.e3.
Barad DH. Use of follicle-stimulating hormone test to predict poor response in in vitro fertilization. Obstet Gynecol. 2005;106:196–7 author reply 197.
Biasoni V, Patriarca A, Dalmasso P, Bertagna A, Manieri C, Benedetto C, et al. Ovarian sensitivity index is strongly related to circulating AMH and may be used to predict ovarian response to exogenous gonadotropins in IVF. Reprod Biol Endocrinol. 2011;9:112.
Bilibio JP, Meireles AJC, Conto ED, Lorenzzoni PL, Nascimento FC. do, Cunha-Filho JS da. GDF9 polymorphisms: influence on ovarian response in women undergoing controlled ovarian hyperstimulation. JBRA Assist Reprod. 2020;24:447–53.
Binder H, Strick R, Zaherdoust O, Dittrich R, Hamori M, Beckmann MW, et al. Assessment of FSHR variants and antimüllerian hormone in infertility patients with a reduced ovarian response to gonadotropin stimulation. Fertil Steril. 2012;97:1169–75.e1.
Burks HR, Ross L, Opper N, Paulson E, Stanczyk FZ, Chung K. Can highly sensitive antimüllerian hormone testing predict failed response to ovarian stimulation? Fertil Steril. 2015;104:643–8.
Buyuk E, Seifer DB, Younger J, Grazi RV, Lieman H. Random anti-Müllerian hormone (AMH) is a predictor of ovarian response in women with elevated baseline early follicular follicle-stimulating hormone levels. Fertil Steril. 2011;95:2369–72.
Cai J, Lou H-Y, Dong M-Y, Lu X-E, Zhu Y-M, Gao H-J, et al. Poor ovarian response to gonadotropin stimulation is associated with low expression of follicle-stimulating hormone receptor in granulosa cells. Fertil Steril. 2007;87:1350–6.
Cerra C, Oliver J, Roberts SA, Horne G, Newman WG, Mohiyiddeen L. A single nucleotide polymorphism of bone morphogenic protein-15 is not associated with ovarian reserve or response to ovarian stimulation. Hum Reprod. 2014:2832–7.
Cerra C, Newman WG, Tohlob D, Byers H, Horne G, Roberts SA, et al. AMH type II receptor and AMH gene polymorphisms are not associated with ovarian reserve, response, or outcomes in ovarian stimulation. J Assist Reprod Genet. 2016;33:1085–91.
Chambers AE, Fairbairn C, Gaudoin M, Mills W, Woo I, Pandian R, et al. Soluble LH-HCG receptor and oestradiol as predictors of pregnancy and live birth in IVF. Reprod BioMed Online. 2019;38:159–68.
Choi YS, Ku S-Y, Jee B-C, Suh C-S, Choi YM, Kim JG, et al. Comparison of follicular fluid IGF-I, IGF-II, IGFBP-3, IGFBP-4 and PAPP-A concentrations and their ratios between GnRH agonist and GnRH antagonist protocols for controlled ovarian stimulation in IVF-embryo transfer patients. Hum Reprod. 2006;21:2015–21.
Coskun B, Dilbaz B, Karadag B, Coskun B, Tohma YA, Dur R, et al. The role of anti-Mullerian hormone in predicting the response to clomiphene citrate in unexplained infertility. Taiwan J Obstet Gynecol. 2018;57:713–7.
Cunha-Filho JSL, Lemos NA, Freitas FM, Facin AC, Gewher-Filho PE, Passos EP. Insulin-like growth factor-1 and insulin-like growth factor binding protein-1 and 3 in the follicular fluid of infertile patients submitted to in vitro fertilization. J Assist Reprod Genet. 2005;22:207–11.
de Castro F, Ruiz R, Montoro L, Pérez-Hernández D, Sánchez-Casas Padilla E, Real LM, et al. Role of follicle-stimulating hormone receptor Ser680Asn polymorphism in the efficacy of follicle-stimulating hormone. Fertil Steril. 2003;80:571–6.
de Mattos CS, Trevisan CM, Peluso C, Adami F, Cordts EB, Christofolini DM, et al. ESR1 and ESR2 gene polymorphisms are associated with human reproduction outcomes in Brazilian women. J Ovarian Res. 2014;7:114.
Desai SS, Achrekar SK, Paranjape SR, Desai SK, Mangoli VS, Mahale SD. Association of allelic combinations of FSHR gene polymorphisms with ovarian response. Reprod BioMed Online. 2013;27:400–6.
Dorn C, Reinsberg J, Kupka M, van der Ven H, Schild RL, Leptin VEGF. IGF-1, and IGFBP-3 concentrations in serum and follicular fluid of women undergoing in vitro fertilization. Arch Gynecol Obstet. 2003;268:187–93.
Ficicioglu C, Cenksoy PO, Yildirim G, Kaspar C. Which cut-off value of serum anti-Müllerian hormone level can predict poor ovarian reserve, poor ovarian response to stimulation and in vitro fertilization success? A prospective data analysis. Gynecol Endocrinol. 2014;30:372–6.
Fried G, Remaeus K, Harlin J, Krog E, Csemiczky G, Aanesen A, et al. Inhibin B predicts oocyte number and the ratio IGF-I/IGFBP-1 may indicate oocyte quality during ovarian hyperstimulation for in vitro fertilization. J Assist Reprod Genet. 2003;20:167–76.
Ganidou MA, Kolibianakis EM, Venetis CA, Gerou S, Makedos GA, Klearchou N, et al. Is assessment of anti-mullerian hormone and/or antral follicle count useful in the prediction of ovarian response in expected normal responders treated with a fixed dose of recombinant FSH and GnRH antagonists? A prospective observational study. Gynecol Endocrinol. 2014;30:817–21.
Genc G, Yilmaz N, Uygur D, Dogan M, Mollamahmutoglu L. The effect of intrafollicular IGF 1 and IGFBP 3 on IVF outcome in patients using different gonadotropins: a prospective study. J Assist Reprod Genet. 2011;28:405–10.
Grzegorczyk-Martin V, Khrouf M, Bringer-Deutsch S, Mayenga J-M, Kulski O, Cohen-Bacrie P, et al. Low circulating anti-Müllerian hormone and normal follicle stimulating hormone levels: which prognosis in an IVF program? Gynecol Obstet Fertil. 2012;40:411–8.
Gürbüz B, Yalti S, Ficicioglu C, Taşdemir S. The relation of serum and follicular fluid leptin and ovarian steroid levels in response to induction of ovulation in in vitro fertilization cycles. Eur J Obstet Gynecol Reprod Biol. 2005;118:214–8.
Hamdine O, Eijkemans MJC, Lentjes EWG, Torrance HL, Macklon NS, Fauser BCJM, et al. Ovarian response prediction in GnRH antagonist treatment for IVF using anti-Müllerian hormone. Hum Reprod. 2015;30:170–8.
Hanevik HI, Hilmarsen HT, Skjelbred CF, Tanbo T, Kahn JA. Single nucleotide polymorphisms in the anti-Müllerian hormone signalling pathway do not determine high or low response to ovarian stimulation. Reprod BioMed Online. 2010;21:616–23.
Hanevik HI, Hilmarsen HT, Skjelbred CF, Tanbo T. Kahn JA. A single nucleotide polymorphism in BMP15 is associated with high response to ovarian stimulation. Reprod BioMed Online. 2011;23:97–104.
Hanevik HI, Hilmarsen HT, Skjelbred CF, Tanbo T, Kahn JA. Variant-beta luteinizing hormone is not associated with poor ovarian response to controlled ovarian hyperstimulation. Reprod Biol Endocrinol. 2014;12:20.
Hatzi E, Bouba I, Galidi A, Lazaros L, Xita N, Sakaloglou P, et al. Association of serum and follicular fluid SHBG levels and SHBG (TAAAA)n polymorphism with follicle size in women undergoing ovarian stimulation. Gynecol Endocrinol. 2011;27:27–32.
Hauzman EE, Lagarde AR, Nagy K, Fancsovits P, Murber A, Jánoki G, et al. Prognostic value of serum CA-125 measurements on stimulation day 1 and on the day of oocyte pickup in the prediction of IVF treatment outcome. J Assist Reprod Genet. 2005;22:265–8.
Huang X, Li L, Hong L, Zhou W, Shi H, Zhang H, et al. The Ser680Asn polymorphism in the follicle-stimulating hormone receptor gene is associated with the ovarian response in controlled ovarian hyperstimulation. Clin Endocrinol. 2015;82:577–83.
Iwase A, Ando H, Kuno K, Mizutani S. Use of follicle-stimulating hormone test to predict poor response in in vitro fertilization. Obstet Gynecol. 2005;105:645–52.
Kahyaoglu S, Yumusak OH, Ozgu-Erdinc AS, Yilmaz S, Kahyaoglu I, Engin-Ustun Y, et al. Can serum estradiol levels on the fourth day of IVF/ICSI cycle predict outcome in poor responder women? Syst Biol Reprod Med. 2015;61:233–7.
Karakaya C, Guzeloglu-Kayisli O, Uyar A, Kallen AN, Babayev E, Bozkurt N, et al. Poor ovarian response in women undergoing in vitro fertilization is associated with altered microRNA expression in cumulus cells. Fertil Steril. 2015;103:1469–76.e1–3.
Karakaya C, Guzeloglu-Kayisli O, Hobbs RJ, Gerasimova T, Uyar A, Erdem M, et al. Follicle-stimulating hormone receptor (FSHR) alternative skipping of exon 2 or 3 affects ovarian response to FSH. MHR: Basic Sci Reproduct Med. 2014;20:630–43.
Kaviani M, Ghaderian SMH, Arefi S, Hashemi M, Afjeh SSA. Role of FSHR rs6165 and ESR2 rs4986938 polymorphisms in ovarian stimulation of Iranian women who underwent assisted reproduction treatment. Hum Antibodies. 2017;26:121–6.
Knez J, Kovačič B, Medved M, Vlaisavljević V. What is the value of anti-Müllerian hormone in predicting the response to ovarian stimulation with GnRH agonist and antagonist protocols? Reprod Biol Endocrinol. 2015;13:58.
Kunt C, Ozaksit G, Kurt RK, Gungor ANC, Kanat-Pektas M, Kilic S, et al. Anti-Mullerian hormone is a better marker than inhibin B, follicle stimulating hormone, estradiol or antral follicle count in predicting the outcome of in vitro fertilization. Arch Gynecol Obstet. 2011;283:1415–21.
Lazaros L, Hatzi E, Xita N, Takenaka A, Sofikitis N, Zikopoulos K, et al. Influence of FSHR diplotypes on ovarian response to standard gonadotropin stimulation for IVF/ICSI. J Reprod Med. 2013;58:395–401.
Lazaros LA, Hatzi EG, Pamporaki CE, Sakaloglou PI, Xita NV, Markoula SI, et al. The ovarian response to standard gonadotrophin stimulation depends on FSHR, SHBG and CYP19 gene synergism. J Assist Reprod Genet. 2012;29:1185–91.
Vagnini LD, Renzi A, Oliveira-Pelegrin GR, do Carmo Tomitão Canas M, Petersen CG, Mauri AL, et al. The TP73 gene polymorphism (rs4648551, A>G) is associated with diminished ovarian reserve. PLoS ONE. 2015;10:e0120048.
Lee RKK, Wu FSY, Lin M-H, Lin S-Y, Hwu Y-M. The predictability of serum anti-Müllerian level in IVF/ICSI outcomes for patients of advanced reproductive age. Reprod Biol Endocrinol. 2011;9:115.
Li R, Gong F, Zhu Y, Fang W, Yang J, Liu J, et al. Anti-Müllerian hormone for prediction of ovarian response in Chinese infertile women undergoing IVF/ICSI cycles: a prospective, multi-centre, observational study. Reprod BioMed Online. 2016;33:506–12.
Liang X, Zhuang G, Zhou C. The predication of ovarian response in control ovarian hyperstimulation by the ratio of basal FSH and LH level. Zhonghua Yi Xue Za Zhi. 2001;81:819–21.
Lledó B, Llácer J, Turienzo A, Ortiz JA, Guerrero J, Morales R, et al. Androgen receptor CAG repeat length is associated with ovarian reserve but not with ovarian response. Reprod BioMed Online. 2014:509–15.
Loutradis D, Patsoula E, Minas V, Koussidis GA, Antsaklis A, Michalas S, et al. FSH receptor gene polymorphisms have a role for different ovarian response to stimulation in patients entering IVF/ICSI-ET programs. J Assist Reprod Genet. 2006;23:177–84.
Morón FJ, de Castro F, Royo JL, Montoro L, Mira E, Sáez ME, et al. Bone morphogenetic protein 15 (BMP15) alleles predict over-response to recombinant follicle stimulation hormone and iatrogenic ovarian hyperstimulation syndrome (OHSS). Pharmacogenet Genomics. 2006;16:485–95.
Motawi TMK, Rizk SM, Maurice NW, Maged AM, Raslan AN, Sawaf AH. The role of gene polymorphisms and AMH level in prediction of poor ovarian response in Egyptian women undergoing IVF procedure. J Assist Reprod Genet. 2017;34:1659–66.
Muttukrishna S, McGarrigle H, Wakim R, Khadum I, Ranieri DM, Serhal P. Antral follicle count, anti-mullerian hormone and inhibin B: predictors of ovarian response in assisted reproductive technology? BJOG. 2005;112:1384–90.
Muttukrishna S, Suharjono H, McGarrigle H, Sathanandan M, Inhibin B. anti-Mullerian hormone: markers of ovarian response in IVF/ICSI patients? BJOG. 2004;111:1248–53.
Neulen J, Wenzel D, Hornig C, Wünsch E, Weissenborn U, Grunwald K, et al. Poor responder-high responder: the importance of soluble vascular endothelial growth factor receptor 1 in ovarian stimulation protocols. Hum Reprod. 2001;16:621–6.
Ng EHY, Chan CCW, Tang OS, Ho PC. Antral follicle count and FSH concentration after clomiphene citrate challenge test in the prediction of ovarian response during IVF treatment. Hum Reprod. 2005;20:1647–54.
Nikolettos N, Asimakopoulos B, Nicolettos N, Efthimiadou A, Mourvati E, Demirel C. Evaluation of leptin, interleukin-1beta, tumor necrosis factor-alpha and vascular endothelial growth factor in serum and follicular fluids of women undergoing controlled ovarian hyperstimulation as prognostic markers of ICSI outcome. In Vivo. 2004;18:667–73.
Nordqvist S, Kårehed K, Skoog Svanberg A, Menezes J, Åkerud H. Ovarian response is affected by a specific histidine-rich glycoprotein polymorphism: a preliminary study. Reprod BioMed Online. 2015;30:74–81.
O’Brien TJ, Kalmin MM, Harralson AF, Clark AM, Gindoff I, Simmens SJ, et al. Association between the luteinizing hormone/chorionic gonadotropin receptor (LHCGR) rs4073366 polymorphism and ovarian hyperstimulation syndrome during controlled ovarian hyperstimulation. Reprod Biol Endocrinol. 2013;11:71.
O’Brien TJ, Harralson AF, Tran T, Gindoff I, Orkunoglu-Suer FE, Frankfurter D, et al. Kinase insert domain receptor/vascular endothelial growth factor receptor 2 (KDR) genetic variation is associated with ovarian hyperstimulation syndrome. Reprod Biol Endocrinol. 2014;12:36.
Pavlik R, Hecht S, Ochsenkühn R, Noss U, Lohse P, Thaler CJ. Divergent effects of the 677C>T mutation of the 5,10-methylenetetrahydrofolate reductase (MTHFR) gene on ovarian responsiveness and anti-Müllerian hormone concentrations. Fertil Steril. 2011;95:2257–62.
Peluso C, Goldman C, Cavalcanti V, Gastaldo G, Trevisan CM, Christofolini DM, et al. Use of bone morphogenetic protein 15 polymorphisms to predict ovarian stimulation outcomes in infertile Brazilian women. Genet Test Mol Biomarkers. 2017;21:328–33.
Qin Y, Zhao Z, Sun M, Geng L, Che L, Chen Z-J. Association of basal serum testosterone levels with ovarian response and in vitro fertilization outcome. Reprod Biol Endocrinol. 2011;9:9.
Quintana R, Kopcow L, Marconi G, Sueldo C, Speranza G, Barañao RI. Relationship of ovarian stimulation response with vascular endothelial growth factor and degree of granulosa cell apoptosis. Hum Reprod. 2001;16:1814–8.
Riggs R, Kimble T, Oehninger S, Bocca S, Zhao Y, Leader B, et al. Anti-Müllerian hormone serum levels predict response to controlled ovarian hyperstimulation but not embryo quality or pregnancy outcome in oocyte donation. Fertil Steril. 2011;95:410–2.
Romão GS, de AS NPA, Ferriani RA, Dib LA, Rodrigues J, Bortolieiro MAV. Serum anti-Müllerian hormone to predict ovarian response in assisted reproduction cycles. Rev Bras Ginecol Obstet. 2012;34:575–81.
Ruiz-Sanz J-I, Pérez-Ruiz I, Meijide S, Ferrando M, Larreategui Z, Ruiz-Larrea M-B. Lower follicular n-3 polyunsaturated fatty acid levels are associated with a better response to ovarian stimulation. J Assist Reprod Genet. 2019;36:473–82.
Scalici E, Bechoua S, Astruc K, Duvillard L, Gautier T, Drouineaud V, et al. Apolipoprotein B is regulated by gonadotropins and constitutes a predictive biomarker of IVF outcomes. Reprod Biol Endocrinol. 2016;14:28.
Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum müllerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77:468–71.
Shrim A, Elizur SE, Seidman DS, Rabinovici J, Wiser A, Dor J. Elevated day 3 FSH/LH ratio due to low LH concentrations predicts reduced ovarian response. Reprod BioMed Online. 2006:418–22.
Siddiqui QUA, Anjum S, Zahra F, Yousuf SM. Ovarian reserve parameters and response to controlled ovarian stimulation in infertile patients. Pak J Med Sci Q. 2019;35:958–62.
Singh N, Malik E, Banerjee A, Chosdol K, Sreenivas V, Mittal S. “Anti-Mullerian hormone: marker for ovarian response in controlled ovarian stimulation for IVF patients”: a first pilot study in the Indian population. J Obstet Gynaecol India. 2013;63:268–72.
Smeenk J, Sweep F, Zielhuis G, Kremer J, Thomas C, Braat D. Antimüllerian hormone predicts ovarian responsiveness, but not embryo quality or pregnancy, after in vitro fertilization or intracyoplasmic sperm injection. Fertil Steril. 2007;87:223–6.
Sudo S, Kudo M, Wada S-I, Sato O, Hsueh AJW, Fujimoto S. Genetic and functional analyses of polymorphisms in the human FSH receptor gene. Mol Hum Reprod. 2002;8:893–9.
Tanriverdi G, Denir S, Ayla S, Bilir A, Oktar H, Cepni I, et al. Notch signaling pathway in cumulus cells can be a novel marker to identify poor and normal responder IVF patients. J Assist Reprod Genet. 2013;30:1319–26.
Tohlob D, Abo Hashem E, Ghareeb N, Ghanem M, Elfarahaty R, Byers H, et al. Association of a promoter polymorphism in FSHR with ovarian reserve and response to ovarian stimulation in women undergoing assisted reproductive treatment. Reprod BioMed Online. 2016;33:391–7.
Tolikas A, Tsakos E, Gerou S, Prapas Y, Loufopoulos A. Anti-Mullerian Hormone (AMH) levels in serum and follicular fluid as predictors of ovarian response in stimulated (IVF and ICSI) cycles. Hum Fertil. 2011;14:246–53.
Urbancsek J, Hauzman EE, Murber A, Lagarde AR, Rabe T, Papp Z, et al. Serum CA-125 and inhibin B levels in the prediction of ovarian response to gonadotropin stimulation in in vitro fertilization cycles. Gynecol Endocrinol. 2005;21:38–44.
Wang T-T, Wu Y-T, Dong M-Y, Sheng J-Z, Leung PCK, Huang H-F. G546A polymorphism of growth differentiation factor-9 contributes to the poor outcome of ovarian stimulation in women with diminished ovarian reserve. Fertil Steril. 2010;94:2490–2.
Wu C-H, Chen Y-C, Wu H-H, Yang J-G, Chang Y-J, Tsai H-D. Serum anti-Müllerian hormone predicts ovarian response and cycle outcome in IVF patients. J Assist Reprod Genet. 2009;26:383–9.
Wu Y-T, Wang T-T, Chen X-J, Zhu X-M, Dong M-Y, Sheng J-Z, et al. Bone morphogenetic protein-15 in follicle fluid combined with age may differentiate between successful and unsuccessful poor ovarian responders. Reprod Biol Endocrinol. 2012;10:116.
Xiao S, Li Y, Long L, Luo C, Mai Q. Basal serum testosterone levels correlate with ovarian reserve and ovarian response in cycling women undergoing in vitro fertilization. Gynecol Endocrinol. 2016;32:51–4.
Yan Y, Gong Z, Zhang L, Li Y, Li X, Zhu L, et al. Association of follicle-stimulating hormone receptor polymorphisms with ovarian response in Chinese women: a prospective clinical study. PLoS One. 2013;8:e78138.
Yin Q, Li Y, Huang J, Yang D. Association of rs13405728 polymorphism of LHR gene with slow ovarian response. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2015;32:840–3.
Yoshida Y, Yamashita Y, Saito N, Ono Y, Yamamoto H, Nakamura Y, et al. Analyzing the possible involvement of anti-Müllerian hormone and anti-Müllerian hormone receptor II single nucleotide polymorphism in infertility. J Assist Reprod Genet. 2014;31:163–8.
Zamaniara T, Taheripanah R, Ghaderian SMH, Zamaniara E, Aghabozorgi SSA, Polymorphism FSHR. (-29G/A) as a genetic agent together with ESRI (XbaIG/A) in women with poor response to controlled ovarian hyperstimulation. Hum Antibodies. 2017;26:143–7.
Loutradis D, Theofanakis C, Anagnostou E, Mavrogianni D, Partsinevelos GA. Genetic profile of SNP(s) and ovulation induction. Curr Pharm Biotechnol. 2012;13:417–25.
Čuš M, Vlaisavljević V, Repnik K, Potočnik U, Kovačič B. Could polymorphisms of some hormonal receptor genes, involved in folliculogenesis help in predicting patient response to controlled ovarian stimulation? J Assist Reprod Genet. 2019;36:47–55.
Ramaraju GA, Cheemakurthi R, Prathigudupu K, Balabomma KL, Kalagara M, Thota S, et al. Role of Lh polymorphisms and r-hLh supplementation in GnRh agonist treated ART cycles: A cross sectional study. Eur J Obstet Gynecol Reprod Biol. 2018;222:119–25.
Morón FJ, Ruiz A. Pharmacogenetics of controlled ovarian hyperstimulation: time to corroborate the clinical utility of FSH receptor genetic markers. Pharmacogenomics. 2010;11:1613–8.
McCarthy MI, Abecasis GR, Cardon LR, Goldstein DB, Little J, Ioannidis JPA, et al. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat Rev Genet. 2008;9:356–69.
Anagnostou E, Mavrogianni D, Theofanakis C, Drakakis P, Bletsa R, Demirol A, et al. ESR1, ESR2 and FSH receptor gene polymorphisms in combination: a useful genetic tool for the prediction of poor responders. Curr Pharm Biotechnol. 2012;13:426–34.
Giacomini KM, Brett CM, Altman RB, Benowitz NL, Dolan ME, Flockhart DA, et al. The pharmacogenetics research network: from SNP discovery to clinical drug response. Clin Pharmacol Ther. 2007;81:328–45.
Overbeek A, Lambalk N. Pharmacogenomics of ovulation induction: facilitating decisions on who, when and how to treat. Pharmacogenomics. 2009;10:1377–9.
La Marca A, Giulini S, Tirelli A, Bertucci E, Marsella T, Xella S, et al. Anti-Müllerian hormone measurement on any day of the menstrual cycle strongly predicts ovarian response in assisted reproductive technology. Hum Reprod. 2007;22:766–71.
Shahrokh Tehraninezhad E, Mehrabi F, Taati R, Kalantar V, Aziminekoo E, Tarafdari A. Analysis of ovarian reserve markers (AMH, FSH, AFC) in different age strata in IVF/ICSI patients. Int J Reprod Biomed (Yazd). 2016;14:501–6.
Code availability
Not applicable.
Funding
This research was funded by FIPE-HCPA and CNPq.
Author information
Authors and Affiliations
Contributions
BSE and JSCF were responsible for the planning and execution of the project, data analysis, statistical analysis, and draft of the manuscript. VKG and CB were responsible for revised selected papers and ethical committee approval. JSCF and GCSV performed in silico analysis. VKG and RD revised the manuscript and participated in the final redaction.
Corresponding author
Ethics declarations
Ethics approval
Approval was obtained from the ethics committee of Hospital de Clinicas de Porto Alegre (IRB-eq). The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eisele, B.S., Silva, G.C.V., Bessow, C. et al. An in silico model using prognostic genetic factors for ovarian response in controlled ovarian stimulation: A systematic review. J Assist Reprod Genet 38, 2007–2020 (2021). https://doi.org/10.1007/s10815-021-02141-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-021-02141-0