Abstract
Purpose
The main purpose and research question of the study are to compare the efficacy of high-security closed versus open devices for human oocytes’ vitrification.
Methods
A prospective randomized study was conducted. A total of 737 patients attending the Infertility and IVF Unit at S.Orsola University Hospital (Italy) between October 2015 and April 2020 were randomly assigned to two groups. A total of 368 patients were assigned to group 1 (High-Security Vitrification™ - HSV) and 369 to group 2 (Cryotop® open system). Oocyte survival, fertilization, cleavage, pregnancy, implantation, and miscarriage rate were compared between the two groups.
Results
No statistically significant differences were observed on survival rate (70.3% vs. 73.3%), fertilization rate (70.8% vs. 74.9%), cleavage rate (90.6% vs. 90.3%), pregnancy/transfer ratio (32.0% vs. 31.8%), implantation rate (19.7% vs. 19.9%), nor miscarriage rates (22.1% vs. 21.5%) between the two groups. Women’s mean age in group 1 (36.18 ± 3.92) and group 2 (35.88 ± 3.88) was not significantly different (P = .297). A total of 4029 oocytes were vitrified (1980 and 2049 in groups 1 and 2 respectively). A total of 2564 were warmed (1469 and 1095 in groups 1 and 2 respectively). A total of 1386 morphologically eligible oocytes were inseminated by intracytoplasmic sperm injection (792 and 594 respectively, P = .304).
Conclusions
The present study shows that the replacement of the open vitrification system by a closed one has no impact on in vitro and in vivo survival, development, pregnancy and implantation rate. Furthermore, to ensure safety, especially during the current COVID-19 pandemic, the use of the closed device eliminates the potential samples’ contamination during vitrification and storage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oocyte storage is used to preserve fertility for medical or social reasons [1,2,3,4,5] and to avoid embryo cryopreservation due to ethical, legal, and moral reasons [6,7,8]. It has also become a valuable tool for egg donor banks [9,10,11,12,13,14].
The principle of oocytes and embryo vitrification is to fully eliminate ice formation in the medium that contains the sample, in phases of cooling, storage, and warming of the procedure [15]. It can be achieved either by increased cooling and warming rates, or increasing concentration of cryoprotectants. Generally, both approaches are used. Cooling rates may vary according to the applied method, from rapid cooling (around 200 °C/min) to ultrarapid (up to 20,000–100,000 °C/min) [16, 17]. Typically, warming is performed rapidly. High cooling and warming rates may help to avoid chilling injury [18].
The cryoprotectant takes part in lowering the freezing point and in reducing or preventing the formation of ice crystals in aqueous solutions. The addition of cryoprotectants into vitrification solutions results in a significant increase in the degree of cellular dehydration. During the warming procedure, the transfer of the oocytes from a solution containing a high concentration of cryoprotectant to an isotonic solution can also lead to a reverse osmotic shock or over-swelling that could be lethal [19]. Therefore, during oocytes and embryo vitrification, a delicate balance between multiple factors is necessary to succeed.
Very high cooling and warming rates, high cryoprotectant concentrations solutions, and a low volume containing the samples are crucial requirements for success human embryos and oocytes’ vitrification [20,21,22].
Over the years, various authors have described different vitrification protocols. They differ in the type of cryoprotectants used (ethylene glycol, DMSO, PROH and sucrose, Ficoll and trehalose, alone or combined) [21]. To date, the most commonly used protocol for both oocyte and embryo vitrification involves the combination of 15% DMSO, 15% EG, and 0.5 M sucrose in a minimum volume (≤ 1 μL) [23]. Moreover, the authors experimented with different equilibrium and dilution parameters and support methods for cooling, storage, and heating.
Devices can be divided into two main categories: “open” and “closed.” The volume surrounding the samples during vitrification is minimal and similar to both systems (< 1 μL). “Open” vitrification devices (such as unprotected Open Pulled Straw [24], Cryotech [25], Cryolock [26], Cryoleaf [27], Vitri-Inga [28]) allow direct contact of the oocytes with liquid nitrogen (LN2) and usually provide very high cooling (20,000 °C/min) rates. Conversely, closed devices (such as CryoTip [29], High-Security Vitrification straw [30], Cryopette [31], Rapid-i [32], VitriSafe [33], and MicroSecure [34]) are unable to reach this cooling rate, but they have the advantage that samples can be stored completely isolated from the external environment. For this reason, the increasing use of closed vitrification devices for oocytes’ cryopreservation must face some scepticism. Nevertheless, it has been reported that the cooling rate obtained with closed devices generates a comparable survival rate if the warming rate is sufficiently high [35]. Some studies suggest that adequate exposure to cryoprotectant agents, before closed vitrification, and high warming rate can compensate for the reduction in cooling rates caused by the thermal insulation of the sample in closed systems [36,37,38]. Furthermore, in the literature, there are reported vitrification systems that do not require high cooling/heating rates and small volumes to obtain vitrified embryos or oocytes (such as I.C.E. Vitrification, Innovative Cryo Enterprises LLC, Linden, NJ) [39,40,41]. Despite the successful use of “open” carriers for vitrification, direct contact between the sample and LN2 may not be desirable because of the potential risk of cross-contamination between specimens and inadvertent exposure to contaminants present in the tanks [42,43,44]. The use of “closed” vitrification carriers circumvents these risks. Some reports confirm the presence of microorganisms, bacteria, and viruses, which may cause infections in LN2 despite its temperature is − 196.5 °C [43, 45]. During long-term cryopreservation, ice sediment accumulates in storage dewars a risk of microbial contamination to stored samples. Ice accumulates in LN2 by two general processes: ice forming in the atmosphere above an open dewar falls into the vessel and ice forming on cold surfaces of the dewar or inventory system that enters LN2. These ice crystals aggregate and entrap other materials, such as bacteria and fungal spores. Theoretically, if the LN2 and its vapors were contaminated, stored samples could also become contaminated; thus, the LN2 itself can be considered a potential source of pathogens during cryopreservation and long-term storage. This problem has led to serious concerns about the use of open devices in vitrification [46,47,48].
The main purpose and research question of the study are to compare the efficacy of high-security closed versus open devices for human oocytes vitrification. In the case of comparable efficacy, closed systems might offer an additional advantage as the samples are not in direct contact with LN2 and thus avoids hypothetical contamination particularly dangerous especially during and after the COVID-19 pandemic.
Materials and methods
The study was approved by the local ethical committee. All the patients attending the Infertility and IVF Unit, at Sant’Orsola University Hospital (Italy), were stimulated with recombinant follicle-stimulating hormone and gonadotropin-releasing hormone analogues. Monitoring was performed with estradiol serum levels’ determinations and pelvic ultrasounds to measure the diameter of the follicles. Ovulation was triggered by an injection of subcutaneous human chorionic gonadotropin (hCG) when the leading follicle reached 18–20 mm diameter and estradiol serum concentrations were around 200 pg/mL for each mature. Vitrification was performed in the occurrence of supernumerary oocytes, failure of semen production, and risk of ovarian hyperstimulation syndrome. For ethical reasons, the Bologna Infertility and IVF Unit cryopreserve female gametes instead of embryos. For this reason, just a few fresh or warmed oocytes are inseminated each time.
Study design
This prospective randomized study was designed to compare the efficiency of vitrification in open versus closed devices. The primary end-point was the survival rate after warming.
francesca.labriola2@unibo.itA total of 1377 oocytes were needed in each arm assuming a survival rate of 65% for High-Security Vitrification™ and 75% for Cryotop® (α 0.05 and power of 80%), based on the average results previously obtained (data not shown).
A total of 860 patients were recruited considering a mean of 4 oocytes per patient and an average cancellation rate of 20% due to personal choices, lack of ovarian response, no retrieved oocytes, or no supernumerary oocytes.
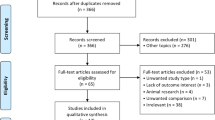
At the end of the randomization process, 737 patients were included in the study, with a final cancellation rate of 14.3% (123 patients). A total of 4029 MII oocytes were vitrified. Oocytes were randomly assigned to the closed group (group 1) or the open group (group 2) (Fig. 1).
Oocyte vitrification method
Denuded oocytes were vitrified, by Vit Kit® (Irvine Scientific), using Kuwayama’s protocol [23]. Oocytes were placed in a pre-balanced 50 μL drop of HEPES solution (37 °C) that was immediately fused with 50 μL of Equilibration Solution (ES) for 3 min, followed by a second fusion with 50 μL of ES for 3 min. Then, the oocytes are transferred to a new 50-μL ES droplet for a variable time of 6–9 min and washed 5 times in vitrification solution (VS) before loading each oocyte on the chosen storage device. Finally, they were plunged in LN2. This procedure must be carried out in 1 min, the time necessary for permeating cryoprotectants to diffuse within the cytoplasm.
Oocyte warming method
Thaw Kit® (Irvine Scientific) was used to warm oocytes according to Kuwayama’s protocol [23]. In the closed vitrification system, the upper extremity of the isolating protective straw was cut and the carrier was quickly submerged in 1 mL preheated thawing solution (TS) without direct contact with LN2. In the open system, the straw cap was initially removed under LN2 where the samples were in direct contact, and the carrier quickly submerged in 1 mL preheated TS. Regardless of the system used, the samples remained in the TS solution for 1 min at 37 °C. Then, oocytes were transferred into a drop of dilution solution (DS) at room temperature (25 °C) and incubated for 3 min. After two subsequent washing procedures in washing solution (WS) at room temperature for 6 min in total, oocytes were transferred into the fertilization medium (Vitrolife). Warmed oocytes were considered morphologically surviving if there were no dark/degenerated or contracted ooplasm and no cracked zona pellucida. Subsequently, oocytes were cultured at 37 °C (6% CO2 and 5% O2). Two hours post warming, oocytes were morphologically re-evaluated. Regardless of semen parameters, intracytoplasmic sperm injection (ICSI) was performed only in non-degenerate oocytes and completely rehydrated. The injected oocytes were cultured in individual 50-μL droplets of cleavage medium (Vitrolife) under oil (Vitrolife). The uninseminated oocytes surviving the warming process have been discarded because they are morphologically not eligible. Oocytes are considered morphologically not eligible if they have one or more of these anomalies: dark zona pellucida, large perivitelline space, dark or granular cytoplasm, cytoplasmic inclusions, vacuoles, and shape abnormalities.
Fertilization and cleavage of warmed oocytes
Fertilization was assessed 16–20 h post ICSI by an inverted microscope (× 20).
Embryo quality was assessed on day 2 using Veeck’s classification [49].
Embryo transfer
Embryos were transferred on day 2. Endometrial preparation was performed with patches containing estradiol hemihydrate: 300 μg/day for 11 days, and when the endometrial thickness reached 10 mm, 600 mg/day of micronized progesterone was added.
Clinical outcomes
Pregnancy was assessed by serum hCG measurement 14–15 days after embryo transfer (value > 5 UI/L) and was then confirmed when at least one gestational sac was visualized at the transvaginal US after two further weeks. Miscarriage was considered the loss of a clinical pregnancy before the 20th week of gestation.
Statistical analysis
Data were expressed as mean ± SD (standard deviation). Student’s t test was used to compare aged, frozen, and warmed oocytes. The chi-square test was used for oocyte’s survival, fertilization, cleavage, number of embryos transferred, pregnancy, implantation, and miscarriage rate. P values < .05 were considered statistically significant. All tests performed were validated through IBM SPSS Statistics (version 25.0).
Results
Tables 1 and 2 show biological and clinical results obtained from vitrification-warming cycles with Hight Security Vitrification™ closed system (group 1) and Cryotop® open system (group 2).
A total of 737 patients for a total of 775 vitrification cycles were analyzed in the present study: 368 (389 vitrification cycles) and 369 (386 vitrification cycles) in groups 1 and 2 respectively.
Women’s age in group 1 (36.18 ± 3.92) and group 2 (35.88 ± 3.88) was not significantly different (P = .297). Women’s range age was 25–46 and 26–44 in groups 1 and 2.
A total of 4029 MII oocytes were vitrified. The closed vitrification system was used for 1980 oocytes, while 2049 were vitrified with the open system. There was no difference in the mean number of oocytes vitrified with the closed or the open system (5.09 ± 3.09 vs. 5.30 ± 3.80) (P = .055). A total of 624 warming cycles (n = 2564) were performed, 354 (n = 1469) and 270 (n = 1095) in groups 1 and 2 respectively. There was no difference in the mean number of warmed oocytes with the closed or the open system (4.15 ± 1.65 vs. 4.06 ± 1.41) (P = .147). Survival rate was 70.3% versus 73.3% (P = .096) in groups 1 and 2 respectively. A total of 1386 oocytes (2.23 ± 0.89) were microinjected, 792 (2.25 ± 0.95) and 594 (2.20 ± 0.82) in groups 1 and 2 respectively (P = .304). Fertilization rate (70.8% vs. 74.9%) and the cleavage rate (90.6% vs. 90.3%) showed no difference between the compared groups.
A total of 297 and 248 transfers were performed in group 1 and 2 respectively. The mean number of embryos transferred was similar in both groups (1.44 ± 0.92 vs. 1.49 ± 0.86) (P = .402). Moreover, there was no statistically significant difference between the closed and open groups in the pregnancy rate/transfer (32.0% vs. 31.8%), in the implantation rate (19.7% vs. 19.9%), or in the miscarriage rate (22.1% vs. 21.5%).
Discussion
The development of a reliable and safe aseptic (closed) vitrification protocol is very important for human cell cryopreservation, especially after the Bielanski reports on the possibility of cross-contamination under LN2 that have raised concern about the use of open vitrification protocols [43,44,45]. So far, the only reported infection in LN2 occurred between blood samples stored in leaky plastic containers [50, 51]. In reproductive biology, however, no single report on disease transmission by LN2-mediated cross-contamination has been published. However, European Parliament’s guidelines (European Parliament and the Council of the European Union, 2004, 2006) have impelled scientists to look for solutions that would maintain vitrification in aseptic [52, 53]. The closed systems were examined and considered potentially harmful for the cells due to the lower cooling rates. At present, considering the increase in fertility preservation treatments, social egg freezing, and oocyte donation banking, storage can be expected to last up to several years. In such prolonged conditions, the main concern is to store biological material as safely as possible. A closed system device might guarantee the safest storage with the appropriate isolation from any detrimental factors [54, 55].
The policy of the University of Bologna Infertility and IVF Unit is to inseminate a limited number of oocytes and avoid the development of supernumerary embryos. Therefore, oocytes’ cryopreservation is offered to most patients.
The present study was able to show that there is no significant difference in survival rate between the devices: 70.3% in the closed HSV device versus 73.3% in the open Cryotop device. When compared to two other prospective randomized studies comparing open and closed devices [56, 57], this is the second report showing no difference in survival rate. Previously, only De Munck’s study [58] showed that there is no difference in survival rate: 93.7% in the closed HSV device versus 89.9% in the open CryotopSC device. Instead, the two previous reports found a significantly lower survival rate for the closed device: 57.9% versus 82.8% [56] and 82.9% versus 91.0% [57]. In the present study, the fertilization rate of the oocytes stored with close systems that survived warming is 70.8%. The result is comparable to the one obtained by De Munck (74.3%) and is in between the fertilization rates obtained by Paffoni (57.6%) and Papatheodorou (82.5%), whereas the fertilization rate of the open device is comparable to that obtained by Paffoni (73.0%) and Papatheodorou (73.4%) and is lower than that obtained by De Munck (81.4%). The degeneration rate after ICSI is not statistically significant between the two groups (6.8% in the closed HSV device vs. 5.9% in the open CryotopSC device). De Munck has observed a significantly higher degeneration rate after ICSI in the HSV arm (11.4% vs. 6.1%) and also Paffoni has observed a high degeneration rate with the closed device, 10.6% versus 6.3%, but it was not significantly different. In the present study, a clinical pregnancy rate of 31.8% in the open device and 32.0% in the closed device was obtained.
These results are in line with the study by Papatheodorou: 36.0% clinical pregnancy rate per cycle in the closed Vitrisafe device and 28.0% in the open Vitrisafe device, while Paffoni’s study reported a very low clinical pregnancy rate per cycle of 7.8% in the closed CryoTip device and 26.4% in the open CryoTop device. In the mentioned reports [57, 58], the average number of embryos transferred was very high (2.81 and 2.79 in Papatheodorou and 1.6 in De Munck). The present study shows a lower number of embryos transferred (1.47) compared to Papatheodorou’s and De Munck’s studies. It is difficult to compare our data with literature reports, as different types of open and closed devices are being used: closed CryoTip versus open CryoTop (Paffoni), closed versus open Vitrisafe device (Papatheodorou). In De Munck’s study only used the closed HSV versus open CryotopSC. Moreover, the study designs were different: the study by Paffoni was a retrospective inter-patient analysis, the study by Papatheodorou was a prospective randomized study where each recipient received oocytes from one type of device, while in the study by De Munck each recipient received oocytes from both devices.
Also, the studies by Papatheodorou and De Munck were conducted on sibling oocyte in a donation program.
On March 11, 2020, the World Health Organization (WHO) declared that the COVID-19 infection became pandemic due to its global geographical distribution [59]. Since then, several international scientific institutions in Reproductive Medicine, including the European Society of Human Reproduction and Embryology (ESHRE), American Society for Reproductive Medicine (ASRM), and Italian National Institute of Health (ISS), suggested suspending initiation of reproductive treatments, as well as fresh/frozen embryo transfers. However, the scientific community highlighted that fertility preservation due to oncological reasons or critical ovarian reserve should be performed storing gametes with the safest procedure [60,61,62,63]. Indeed, the Infertility and IVF Unity at the University of Bologna performed a number of oocyte storage for fertility preservation in cancer patients during the pandemic lockdown.
The results of the present study indicate that the high-security vitrification system is efficient and represents a valid strategy for storing biological samples in the safest way to reduce the potential risk of viral contamination. These results are of pivotal importance since many uncertainties remain around the effects of SARS-CoV-2 infection on ART.
Data Availability
Not applicable
References
Cobo A, Kuwayama M, Pérez S, Ruiz A, Pellicer A, Remohí J. Comparison of concomitant outcome achieved with fresh and cryopreserved donor oocytes vitrified by the Cryotop method. Fertil Steril. 2008.
Homburg R, van der Veen F, Silber SJ. Oocyte vitrification-women’s emancipation set in stone. Fertil Steril. 2009.
Noyes N, Labella PA, Grifo J, Knopman JM. Oocyte cryopreservation: a feasible fertility preservation option for reproductive age cancer survivors. J Assist Reprod Genet. 2010;27:495–9.
Porcu E, Venturoli S, Damiano G, Ciotti PM, Notarangelo L, Paradisi R, Moscarini M, Ambrosini G. Healthy twins delivered after oocyte cryopreservation and bilateral ovariectomy for ovarian cancer. Reprod Biomed Online. 2008.
Levi Setti PE, Porcu E, Patrizio P, Vigiliano V, De Luca R, D’Aloja P, Spoletini R, Scaravelli G. Human oocyte cryopreservation with slow freezing versus vitrification. Results from National Italian Registry data, 2007–2011. Fertil Steril. 2014;102(1):90–5.e2.
Boldt J, Cline D, McLaughlin D. Human oocyte cryopreservation as an adjunct to IVF-embryo transfer cycles. Hum Reprod. 2003;18:1250–5.
Kazem R, Thompson LA, Srikantharajah A, Laing MA, Hamilton MP, Templeton A. Cryopreservation of human oocytes and fertilization by two techniques: in-vitro fertilization and intracytoplasmic sperm injection. Hum Reprod. 1995;10:2650–4.
Ragni G, Allegra A, Anserini P, Causio F, Ferraretti AP, Greco E, et al. The 2004 Italian legislation regulating assisted reproduction technology: a multicentre survey on the results of IVF cycles. Hum Reprod. 2005;20:2224–8.
Cobo A, Meseguer M, Remohí J, Pellicer A. Use of cryo-banked oocytes in an ovum donation programme: a prospective, randomized, controlled, clinical trial. Hum Reprod. 2010;25:2239–46.
Nagy ZP, Chang CC, Shapiro DB, Bernal DP, Elsner CW, Mitchell-Leef D, Toledo AA, Kort HI. Clinical evaluation of the efficiency of an oocyte donation program using egg cryo-banking. Fertil Steril. 2009.
Trokoudes KM, Pavlides C, Zhang X. Comparison outcome of fresh and vitrified donor oocytes in an egg-sharing donation program. Fertil Steril. 2011.
Chian RC, Huang JY, Tan SL, Lucena E, Saa A, Rojas A, et al. Obstetric and perinatal outcome in 200 infants conceived from vitrified oocytes. Reprod Biomed Online. 2008;16:608–10.
Cobo A, Serra V, Garrido N, Olmo I, Pellicer A, Remohí J. Obstetric and perinatal outcome of babies born from vitrified oocytes. Fertil Steril. 2014.
Noyes N, Porcu E, Borini A. Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reprod Biomed Online. 2009;18:769–76.
Rall WF, Fahy GM. Ice-free cryopreservation of mouse embryos at -196 degrees C by vitrification. Nature. 1985;313:573–5.
Rall WF. Cryopreservation of mammalian embryos, gametes, and ovarian tissue. In: Assisted Fertilization and Nuclear Transfer in Mammals, and 1. Humana Press Inc; 2001.
Kasai M, Mukaida T. Cryopreservation of animal and human embryos by vitrification. Reprod BioMed Online. 2004.
Ghetler Y, Yavin S, Shalgi R, Arav A. The effect of chilling on membrane lipid phase transition in human oocytes and zygotes. Hum Reprod. 2005.
Fuller BJ, Bernard AG. Successful in vitro fertilization of mouse oocytes after cryopreservation using glycerol. Cryo-lett. 1984.
Practice Committees of American Society for Reproductive Medicine. Society for Assisted Reproductive Techologies. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013.
Vajta G, Nagy ZP. Are programmable freezers still needed in the embryo laboratory? Review on vitrification. Reprod Biomed Online. 2006.
Arav A. Cryopreservation of oocytes and embryos. Theriogenology. 2014;81:96–102.
Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online. 2005.
Vajta G, Holm P, Kuwayama M, Booth PJ, Jacobsen H, Greve T, et al. Open Pulled Straw (OPS) vitrification: a new way to reduce cryoinjuries of bovine ova and embryos. Mol Reprod Dev. 1998;51:53–8.
Gutnisky C, Alvarez GM, Cetica PD, Dalvit GC. Evaluation of the cryotech vitrification kit for bovine embryos. Cryobiology. 2013.
García JI, Noriega-Portella L, Noriega-Hoces L. Efficacy of oocyte vitrification combined with blastocyst stage transfer in an egg donation program. Human Reprod. 2011.
Chian RC, Huang JY, Gilbert L, Son WY, Holzer H, Cui SJ, Buckett WM, Tulandi T, Tan SL. Obstetric outcomes following vitrification of in vitro and in vivo matured oocytes. Fertil Steril. 2009.
Almodin CG, Minguetti-Camara VC, Paixao CL, Pereira PC. Embryo development and gestation using fresh and vitrified oocytes. Hum Reprod. 2010.
Kuwayama M, Vajta G, Ieda S, Kato O. Comparison of open and closed methods for vitrification of human embryos and the elimination of potential contamination. Reprod. Biomed. Online. 2005;11:608–14.
Abdelhafez F, Xu J, Goldberg J, Desai N. Vitrification in open and closed carriers at different cell stages: assessment of embryo survival, development, dna integrity and stability during vapor phase storage for transport. BMC Biotechnol. 2011;11:29.
Keskintepe L, Agca Y, Bayrak A, Sher G. Efffective human and mouse blastocyst vitrification system: introducing Cryopette. Fertil Steril. 2009;92:S24.
Larman MG, Gardner DK. Vitrification of mouse embryos with super-cooled air. Fertil Steril. 2011;95:1462–6.
Vanderzwalmen P, Ectors F, Grobet L, Prapas Y, Panagiotidis Y, Vanderzwalmen S, et al. Aseptic vitrification of blastocysts from infertile patients, egg donors and after IVM. Reprod. Biomed. Online. 2009;19:700–7.
Schiewe MC. MicroSecure vitrification for oocytes and embryos: optimum simplicity, security and cost and effectiveness combining FDA approved products. J Clin Embryol.
Doyle JO, Richter KS, Lim J, Stillman RJ, Graham JR, Tucker MJ. Successful elective and medically indicated oocyte vitrification and warming for autologous in vitro fertilization, with predicted birth probabilities for fertility preservation according to number of cryopreserved oocytes and age at retrieval. Fertil Steril. 2016.
Vajta G, Kuwayama M. Improving cryopreservation systems. Theriogenology. 2006.
Mazur P, Seki S. Survival of mouse oocytes after being cooled in a vitrification solution to -196°C at 95° to 70,000°C/min and warmed at 610° to 118,000°C/min: A new paradigm for cryopreservation by vitrification. Cryobiology. 2011;62:1–7.
Seki S, Mazur P. Ultra-rapid warming yields high survival of mouse oocytes cooled to -196°c in dilutions of a standard vitrification solution. PLoS One. 2012.
Schiewe MC, Zozula S, Anderson RE, Fahy GM. Validation of microSecure vitrification (μS-VTF) for the effective cryopreservation of human embryos and oocytes. Cryobiology. 2015;71:264–72.
Stachecki JJ, Garrisi J, Sabino S, Caetano JPJ, Wiemer KE, Cohen J. A new safe, simple and successful vitrification method for bovine and human blastocysts. Reprod Biomed Online. 2008.
Nagy ZP, Varghese AC, Agarwal A. In Vitro Fertilization. A Textbook of Current and Emerging Methods and Devices. 2019.
De Munck N, Verheyen G, Van Landuyt L, Stoop D, Van de Velde H. Survival and post-warming in vitro competence of human oocytes after high security closed system vitrification. J Assist Reprod Genet. 2013.
Bielanski A, Nadin-Davis S, Sapp T, Lutze-Wallace C. Viral contamination of embryos cryopreserved in liquid nitrogen. Cryobiology. 2000.
Bielanski A, Bergeron H, Lau PC, Devenish J. Microbial contamination of embryos and semen during long term banking in liquid nitrogen. Cryobiology. 2003.
Bielanski A, Vajta G. Risk of contamination of germplasm during cryopreservation and cryobanking in IVF units. Hum Reprod. 2009.
Morris GJ. The origin, ultrastructure, and microbiology of the sediment accumulating in liquid nitrogen storage vessels. Cryobiology. 2005;50:231–8.
Cobo A, Romero JL, Perez S, de los Santos MJ, Meseguer M, Remohí J. Storage of human oocytes in the vapor phase of nitrogen. Fertil Steril. 2010.
Nagy ZP, Chang CC, Shapiro DB, Bernal DP, Kort HI, Vajta G. The efficacy and safety of human oocyte vitrification. Semin Reprod Med. 2009.
Veeck L, Zaninovic N. An Atlas of Human Blastocysts. New York: Informa Healthcare; 2003.
Tedder RS, Zuckerman MA, Goldstone AH, Hawkins AE, Fielding A, Briggs EM, Irwin D, Blair S, Gorman AM, Patterson KG, et al. Hepatitis B transmission from contaminated cryopreservation tank. Lancet. 1995.
Vajta G, Rienzi L, Ubaldi FM. Open versus closed systems for vitrification of human oocytes and embryos. Reprod Biomed Online. 2015.
Official Journal of the European Union. Directive 2004/23/EC of the European Parliament and of the Council of 31 March 2004 on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32004L0023. Accessed 29 March 2016.
Official Journal of the European Union. Commission Directive 2006/17/EC of 8 February 2006 implementing Directive 2004/23/EC of the European Parliament and of the Council as regards certain technical requirements for the donation, procurement and testing of human tissues and cells. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32006L0017. Accessed 29 March 2016.
Stoop D, De Munck N, Jansen E, Platteau P, Van den Abbeel E, Verheyen G, Devroey P. Clinical validation of a closed vitrification system in an oocyte-donation programme. Reprod Biomed Online. 2012.
Camus A, Clairaz P, Ersham A, Van Kappel A-L, Savić G, Staub C. The Comparison of the Process of Five Different Vitrification Devices. Gynecol Obstet Fertil. 2006.
Paffoni A, Guarneri C, Ferrari S, Restelli L, Nicolosi AE, Scarduelli C, Ragni G. Effects of two vitrification protocols on the developmental potential of human mature oocytes. Reprod Biomed Online. 2011.
Papatheodorou A, Vanderzwalmen P, Panagiotidis Y, Prapas N, Zikopoulos K, Georgiou I, et al. Open versus closed oocyte vitrification system: a prospective randomized sibling-oocyte study. Reprod Biomed Online. 2013 Jun.;26:595–602.
De Munck N, Santos-Ribeiro S, Stoop D, Van de Velde H, Verheyen G. Open versus closed oocyte vitrification in an oocyte donation programme: a prospective randomized sibling oocyte study. Hum Reprod. 2016.
WHO - World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report–51. Published on the WHO website. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10. Date of publication: 11/03/2020.
ESHRE COVID-19 Working Group. Assisted reproduction and COVID-19: A statement from ESHRE for phase 1 - Guidance on fertility services during pandemic. Published on the ESHRE website. Available at: https://www.eshre.eu/Press-Room/ESHRE-News#COVID19WG. Date of publication: 2 April 2020 (last revision 17 April).
ESHRE COVID-19 Working Group. Assisted reproduction and COVID-19. A statement from ESHRE for phase 2 - ESHRE Guidance on recommencing ART treatments. Available at: https://www.eshre.eu/Press-Room/ESHRE-News#COVID19WG. Date of publication: 23 April 2020.
American Society For Reproductive Medicine. Patient Management And Clinical Recommendations During The Coronavirus (Covid-19) Pandemic. Available at: https://www.asrm.org/news-and-publications/covid-19/statements/patient-management-and-clinical-recommendations-during-the-coronavirus-covid-19-pandemic/ Update #1 (March 30, 2020 Through April 13, 2020).
Istituto Superiore di Sanità, Centro Nazionale Trapianti. Misure di prevenzione della trasmissione dell’infezione da nuovo Coronavirus (SARS-CoV-2) in Italia per le cellule riproduttive e i trattamenti di PMA (procreazione medicalmente assistita). Published on the ISS website. Available at: http://old.iss.it/binary/rpma/cont/Prot._605.CNT.2020_Misure_di_prevenzione_SARS_COV2_per_cellule_riproduttive_PMA_Aggiornamento_13.03.2020.pdf. Date of publication: 13/03/2020.
Funding
Open Access funding provided by Alma Mater Studiorum - Università di Bologna.
Author information
Authors and Affiliations
Contributions
Professor Eleonora Porcu participated in study conception, critical discussions, and manuscript revision. Material preparation, data collection, and analysis were performed by Dr. Maria Lucrezia Tranquillo, Dr. Leonardo Notarangelo, Dr. Elena Nardi, and Dr. Giuseppe Damiano. The first draft of the manuscript was written by Professor Eleonora Porcu and Dr. Maria Lucrezia Tranquillo. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethics approval
The study was approved by the local ethical committee.
Consent to participate
Not applicable
Consent for publication
Not applicable
Code availability
Not applicable
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Porcu, E., Tranquillo, M.L., Notarangelo, L. et al. High-security closed devices are efficient and safe to protect human oocytes from potential risk of viral contamination during vitrification and storage especially in the COVID-19 pandemic. J Assist Reprod Genet 38, 681–688 (2021). https://doi.org/10.1007/s10815-021-02062-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-021-02062-y