Abstract
Purpose
To establish a mathematical model for assessing the true ovarian reserve based on the predicted probability of poor ovarian response (POR).
Methods
In this retrospective cohort study, a total of 1523 GnRH-antagonist cycles in 2017 were firstly analyzed. The ovarian responses were calculated based on the number of retrieved oocytes. The continuous variables were converted into categorical variables according to cutoff values generated by the decision tree method. The optimal model was identified using forward stepwise multiple logistic regression with 5-fold cross-validation and further verified its performances using outer validation data.
Results
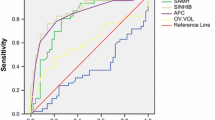
The predictors in our model were anti-Müllerian hormone (AMH), antral follicle counts (AFC), basal follicle-stimulating hormone (FSH), and age, in order of their significance, named AAFA model. The AUC, sensitivity, specificity, positive predictive value, and negative predictive value of AAFA model in inner validation and outer validation data were 0.861 and 0.850, 0.603 and 0.519, 0.917 and 0.930, 0.655 and 0.570, and 0.899 and 0.915. Ovarian reserve of 16 subgroups was further ranked according to the predicted probability of POR and further divided into 4 groups of A–D using clustering analysis. The incidence of POR in the four groups was 0.038 (0.030–0.046), 0.139 (0.101–0.177), 0.362 (0.308–0.415), and 0.571 (0.525–0.616), respectively. The order of ovarian reserve from adequate to poor followed the order of A to D.
Conclusion
We have established an easy applicable AAFA model for assessing true ovarian reserve and may have important implications in both infertile women and general reproductive women in Chinese or Asian population.



Similar content being viewed by others
References
Qiao J, Wang ZB, Feng HL, Miao YL, Wang Q, Yu Y, et al. The root of reduced fertility in aged women and possible therapentic options: current status and future perspects. Mol Asp Med. 2014;38:54–85.
Lass A. Assessment of ovarian reserve: is there still a role for ovarian biopsy in the light of new data? Hum Reprod. 2004;19:467–9.
Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12:685–718.
Wallace WH, Kelsey TW. Human ovarian reserve from conception to the menopause. PLoS One. 2010;5:e8772.
Roudebush WE, Kivens WJ, Mattke JM. Biomarkers of ovarian reserve. Biomark Insights. 2008;3:259–68.
Gynecologists TACoOa. Committee opinion no. 618: ovarian reserve testing. Obstet Gynecol. 2015;125:268–73.
Medicine PCotASfR. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2015;103:e9–e17.
de Carvalho BR, Rosa e Silva AC, Rosa e Silva JC, dos Reis RM, Ferriani RA, Silva de Sa MF. Ovarian reserve evaluation: state of the art. J Assist Reprod Genet. 2008;25:311–22.
Broer SL, van Disseldorp J, Broeze KA, Dolleman M, Opmeer BC, Bossuyt P, et al. Added value of ovarian reserve testing on patient characteristics in the prediction of ovarian response and ongoing pregnancy: an individual patient data approach. Hum Reprod Update. 2013;19:26–36.
Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, et al. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143:1076–84.
Eldar-Geva T, Ben-Chetrit A, Spitz IM, Rabinowitz R, Markowitz E, Mimoni T, et al. Dynamic assays of inhibin B, anti-Mullerian hormone and estradiol following FSH stimulation and ovarian ultrasonography as predictors of IVF outcome. Hum Reprod. 2005;20:3178–83.
van Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, de Jong FH, et al. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83:979–87.
Yding Andersen C. Inhibin-B secretion and FSH isoform distribution may play an integral part of follicular selection in the natural menstrual cycle. Mol Hum Reprod. 2017;23:16–24.
Santoro N. The menopausal transition. Am J Med. 2005;118(Suppl 12B):8–13.
Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L. ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26:1616–24.
Alviggi C, Andersen CY, Buehler K, Conforti A, De Placido G, Esteves SC, et al. A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil Steril. 2016;105:1452–3.
Johnson MKK. Applied predictive modeling, measuring performance in regresssion model. Berlin: Springer; 2013.
Xu H, Zeng L, Yang R, Feng Y, Li R, Qiao J. Retrospective cohort study: AMH is the best ovarian reserve markers in predicting ovarian response but has unfavorable value in predicting clinical pregnancy in GnRH antagonist protocol. Arch Gynecol Obstet. 2017;295:763–70.
Qiao J, Wang ZB, Feng HL, Miao YL, Wang Q, Yu Y, et al. The root of reduced fertility in aged women and possible therapentic options: current status and future perspects. Mol Asp Med. 2013;38:54–85.
Heidar Z, Bakhtiyari M, Mirzamoradi M, Zadehmodarres S, Sarfjoo FS, Mansournia MA. Prediction of different ovarian responses using anti-Mullerian hormone following a long agonist treatment protocol for IVF. J Endocrinol Investig. 2015;38:1007–15.
La Marca A, Sunkara SK. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Hum Reprod Update. 2014;20:124–40.
Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Mullerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril. 2010;93:855–64.
Muttukrishna S, Suharjono H, McGarrigle H, Sathanandan M. Inhibin B and anti-Mullerian hormone: markers of ovarian response in IVF/ICSI patients? Bjog-an Int J Obstetrics Gynaecol. 2004;111:1248–53.
Kotanidis L, Nikolettos K, Petousis S, Asimakopoulos B, Chatzimitrou E, Kolios G, et al. The use of serum anti-Mullerian hormone (AMH) levels and antral follicle count (AFC) to predict the number of oocytes collected and availability of embryos for cryopreservation in IVF. J Endocrinol Investig. 2016;39:1459–64.
La Marca A, Argento C, Sighinolfi G, Grisendi V, Carbone M, D'Ippolito G, et al. Possibilities and limits of ovarian reserve testing in ART. Curr Pharm Biotechnol. 2012;13:398–408.
Medicine TPCotASfR. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2012;98:1407–15.
Riggs R, Kimble T, Oehninger S, Bocca S, Zhao Y, Leader B, et al. Anti-Mullerian hormone serum levels predict response to controlled ovarian hyperstimulation but not embryo quality or pregnancy outcome in oocyte donation. Fertil Steril. 2011;95:410–2.
Nardo LG, Gelbaya TA, Wilkinson H, Roberts SA, Yates A, Pemberton P, et al. Circulating basal anti-Mullerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril. 2009;92:1586–93.
Funding
This study was supported by the National Key Research and Development Program of China (Grant No. 2018YFC1002100, 2016YFC1000302, 2016YFC1000201); the capital health research and development of special project (Grant No. 2018-1-4091); National Natural Science Foundation of China (Grant No. 81771650); and Major National R&D Projects of China (Grant No. 2017ZX09304012-012).
Author information
Authors and Affiliations
Contributions
Huiyu Xu: data collection and manuscript writing. Guoshang Feng: statistical analysis and manuscript writing. Haiyan Wang: data collection and manuscript writing. Yong Han: editing of this manuscript. Rui Yang: data collection and clinical consultation. Ying song: data collection and clinical consultation. Lixue Chen: data collection. Li Shi: data collection. Mengqian Zhang: data collection. Rong Li: resources, study design, supervision, and finally manuscript approval. Jie Qiao: resources, study design
Corresponding author
Ethics declarations
The dataset used in this study contains de-identified data; thus, the informed consent by the patients was waived and the institutional review board approval was exempted, which conform to the Helsinki declaration.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, H., Feng, G., Wang, H. et al. A novel mathematical model of true ovarian reserve assessment based on predicted probability of poor ovarian response: a retrospective cohort study. J Assist Reprod Genet 37, 963–972 (2020). https://doi.org/10.1007/s10815-020-01700-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-01700-1