Abstract
Purpose
To compare chromosomal aberrations and aneuploidy features in (i) blastocysts following intracytoplasmic sperm injection (ICSI) and trophectoderm (TE) biopsy using preimplantation genetic screening (PGS) and (ii) early spontaneous abortion chorionic villus biopsies (SA-CVB) using single-nucleotide polymorphism (SNP) array detection.
Methods
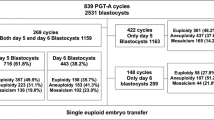
We retrospectively reviewed the data for 1014 TEs from 220 PGS cycles and 1724 SA-CVBs originating from naturally pregnant couples and patients undergoing assisted reproductive technology (ART) during 2017 to 2018. SNP array was applied in both PGS and SA-CVBs detection. Aberrations were defined, and the frequency and ratio of each chromosome aberration were compared between the two groups.
Results
There were more abnormalities in TEs in the form of complex chromosome aneuploidies and monosomies, while SA-CVBs had more trisomies, sex chromosome abnormalities, and polyploidies. In both groups, chromosomal aneuploidies (including monosomies and trisomies) were confined to chromosomes 14, 15, 16, 18, 21, and 22, but showed varying distributions across the groups. Aneuploidy of chromosome 22 was most frequent in TEs, whereas that of chromosome 16 predominated in SA-CVBs. Among the sex chromosome abnormalities, X monosomies were significantly more prevalent in SA-CVBs.
Conclusions
Chromosomal aberrations and aneuploidy manifested specific characteristics that differed between TEs and SA-CVBs, which indicates that distinct chromosomal abnormalities can affect certain developmental stages of embryos. Further analysis is needed to explore the chromosomal mechanisms affecting embryo development and implantation. Such information will help clinical assessments in prenatal diagnosis and reduce the incidence of genetically abnormal fetuses.





Similar content being viewed by others
References
Lejeune J, Gautier M, Turpin R. Study of somatic chromosomes from 9 mongoloid children. C R Hebd Seances Acad Sci. 1959;248(11):1721–2.
Jacobs PA, Baikie AG, Court BW, Strong JA. The somatic chromosomes in mongolism. Lancet. 1959;1(7075):710.
Rodriguez PJ, Lee J, Whitehouse M, Moschini RM, Knopman J, Duke M, et al. Embryo selection versus natural selection: how do outcomes of comprehensive chromosome screening of blastocysts compare with the analysis of products of conception from early pregnancy loss (dilation and curettage) among an assisted reproductive technology population? Fertil Steril. 2015;104(6):1460–6.
Rubio C, Rodrigo L, Mercader A, Mateu E, Buendia P, Pehlivan T, et al. Impact of chromosomal abnormalities on preimplantation embryo development. Prenat Diagn. 2007;27(8):748–56.
Mantzouratou A, Mania A, Fragouli E, Xanthopoulou L, Tashkandi S, Fordham K, et al. Variable aneuploidy mechanisms in embryos from couples with poor reproductive histories undergoing preimplantation genetic screening. Hum Reprod. 2007;22(7):1844–53.
Spinella F, Fiorentino F, Biricik A, Bono S, Ruberti A, Cotronea E, et al. Extent of chromosomal mosaicism influences the clinical outcome of in vitro fertilization treatments. Fertil Steril. 2018;109(1):77–83.
Capalbo A, Hoffmann ER, Cimadomo D, Ubaldi FM, Rienzi L. Human female meiosis revised: new insights into the mechanisms of chromosome segregation and aneuploidies from advanced genomics and time-lapse imaging. Hum Reprod Update. 2017;23(6):706–22.
Guo LY, Zhai M, Wang SS, Zhang Y. Meta-analysis of risk factors related to the induction of embryo damage. CJCHC FEB. 2016;24(2):166–9.
Christianson RE, Sherman SL, Torfs CP. Maternal meiosis II nondisjunction in trisomy 21 is associated with maternal low socioeconomic status. Genet Med. 2004;6(6):487–94.
Hunter JE, Allen EG, Shin M, Bean LJ, Correa A, Druschel C, et al. The association of low socioeconomic status and the risk of having a child with down syndrome: a report from the National Down Syndrome Project. Genet Med. 2013;15(9):698–705.
Chris MJ, Servi JC, Joseph CD, Marion D, Hubert JS, Bertien HC, et al. SNP array-based copy number and genotype analyses for preimplantation genetic diagnosis of human unbalanced translocations. Eur J Hum Genet. 2012;20(9):938–44.
Elias MD, Jacques B, François A, Douglas W, Jo-Ann B, Carla C, et al. Technical update: preimplantation genetic diagnosis and screening. J Obstet Gynaecol Can2015;37(5):451–463.
Wang Y, Cheng Q, Meng L, Luo C, Hu H, Zhang J, et al. Clinical application of SNP array analysis in first-trimester pregnancy loss: a prospective study. Clin Genet. 2017;91(6):849–58.
Huang J, Li R, Lian Y, Chen L, Shi X, Qiao J, et al. Vitrified/warmed single blastocyst transfer in preimplantation genetic diagnosis/preimplantation genetic screening cycles. Int J Clin Exp Med. 2015;8(11):21605–10.
Doubilet PM, Benson CB, Bourne T, Blaivas M. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369(15):1443–51.
Huang J, Zhao N, Wang X, Qiao J, Liu P. Chromosomal characteristics at cleavage and blastocyst stages from the same embryos. J Assist Reprod Genet. 2015;32(5):781–7.
Taranissi M, El-Toukhy T, Gorgy A. Influence of maternal age on the outcome of PGD for aneuploidy screening in patients with recurrent implantation failure. Reprod BioMed Online. 2005;10(5):628–32.
Findikli N, Kahraman S, Saglam Y, Beyazyurek C, Sertyel S, Karlikaya G, et al. Embryo aneuploidy screening for repeated implantation failure and unexplained recurrent miscarriage. Reprod BioMed Online. 2006;13(1):38–46.
Fragouli E, Alfarawati S, Spath K, Jaroudi S, Sarasa J, Enciso M, et al. The origin and impact of embryonic aneuploidy. Hum Genet. 2013;132(9):1001–13.
Dai XY, Zhou L, Xie JS. Chromosome abnormality analysis of 7036 chorionic villi in spontaneous miscarriage cases by multiplex ligation-dependent probe amplification. Chin J Lab Med. 2017;40(8):598–601.
McCallie BR, Parks JC, Patton AL, Griffin DK, Schoolcraft WB, Katz-Jaffe MG, et al. Hypomethylation and genetic instability in monosomy blastocysts may contribute to decreased implantation potential. PLoS One. 2016;11(7):e159507.
Halloran KH, Breg WR. Mahoney MJ.21 monosomy in a retarded female infant. J Med Genet. 1974;11(4):386–9.
Licciardi F, Lhakhang T, Kramer YG, Zhang Y, Heguy A, Tsirigos A. Human blastocysts of normal and abnormal karyotypes display distinct transcriptome profiles. Nature. 2018;8(1):14906.
Chou ST, Opalinska JB, Yao Y, Fernandes MA, Kalota A, Brooks JS, et al. Trisomy 21 enhances human fetal erythro-megakaryocytic development. BLOOD. 2008;112(12):4503–6.
Munne S, Bahce M, Sandalinas M, Escudero T, Marquez C, Velilla E, et al. Differences in chromosome susceptibility to aneuploidy and survival to first trimester. Reprod BioMed Online. 2004;8(1):81–90.
Fragouli E, Wells D. Aneuploidy in the human blastocyst. Cytogenet Genome Res. 2011;133(2–4):149–59.
Hassold T, Chen N, Funkhouser J, Jooss T, Manuel B, Matsuura J, et al. A cytogenetic study of 1000 spontaneous abortions. Ann Hum Genet. 1980;44(2):151–78.
Du Y, Chen L, Lin J, Zhu J, Zhang N, Qiu X, et al. Chromosomal karyotype in chorionic villi of recurrent spontaneous abortion patients. Biosci Trends. 2018;12(1):32–9.
Emanuel BS, Zackai EH, Aronson MM, Mellman WJ, Moorhead PS. Abnormal chromosome 22 and recurrence of trisomy-22 syndrome. J Med Genet. 1976;13(6):501–6.
Hall HE, Surti U, Hoffner L, Shirley S, Feingold E, Hassold T. The origin of trisomy 22: evidence for acrocentric chromosome-specific patterns of nondisjunction. Am J Med Genet A. 2007;143A:2249–55.
Hassold TJ, Jacobs PA. Trisomy in man. Annu Rev Genet. 1984;18:69–97.
Micale M, Insko J, Ebrahim SA, Adeyinka A, Runke C, Van Dyke DL. Double trisomy revisited--a multicenter experience. Prenat Diagn. 2010;30(2):173–6.
Babariya D, Fragouli E, Alfarawati S, Spath K, Wells D. Hum Reprod. 2017;32(12):2549–60.
McWeeney DT, Munne S, Miller RC, Cekleniak NA, Contag SA, Wax JR, et al. Pregnancy complicated by triploidy: a comparison of the three karyotypes. Am J Perinatol. 2009;26(9):641–5.
Sundvall L, Lund H, Niemann I, Jensen UB, Bolund L, Sunde L. Tetraploidy in hydatidiform moles. Hum Reprod. 2013;28(7):2010–20.
Mathur A, Stekol L, Schatz D, NK ML, Scott ML, Lippe B. The parental origin of the single X chromosome in Turner syndrome: lack of correlation with parental age or clinical phenotype. Am J Hum Genet. 1991;48(4):682–6.
Funding
This study was supported by 2018YFC1003104 “National Key R&D Program of China” and BYSY2018015 “Clinical Key Program of Peking University Third Hospital”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, X., Wang, Y., Zhao, N. et al. Variations in chromosomal aneuploidy rates in IVF blastocysts and early spontaneous abortion chorionic villi. J Assist Reprod Genet 37, 527–537 (2020). https://doi.org/10.1007/s10815-019-01682-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-019-01682-9