Abstract
Purpose
To determine the application value of next-generation sequencing (NGS)-based preimplantation genetic testing for aneuploidies (PGT-A).
Methods
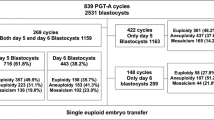
We conducted a retrospective case–control study on a cohort of frozen-thawed embryo transfer (FET) cycles following preimplantation genetic testing for monogenic disorders (PGT-M) between 2014 and 2017. Cycles that produced live births or early miscarriages were divided into live birth group (n = 76) or miscarriage group (n = 19), respectively. The NGS-based aneuploidy screening was performed on the multiple displacement amplification (MDA) products of the embryonic trophectoderm biopsy samples that were cryopreserved following PGT-M.
Results
In the live birth group, 75% (57/76) embryos were euploid and 14.5% (11/76) were aneuploid. The remaining 10.5% (8/76) embryos were NGS-classified mosaic with the high- (≥ 50%) and low-level (< 50%) mosaicism rates at 7.9% (6/76) and 2.6% (2/76), respectively. In the miscarriage group, only 23.5% (4/17) embryos were aneuploid, while 58.8% (10/17) were euploid and 17.6% (3/17) were NGS-classified mosaic with the high- and low-level mosaicism rates at 11.8% (2/17) and 5.9% (1/17), respectively. For live birth and miscarriage groups, the transferable rate was 82.9% (63/76) and 70.6% (12/17), respectively, whereas the untransferable rate was 17.1% (13/76) and 29.4% (5/17), respectively.
Conclusion
The application of NGS-based PGT-A remains questionable, as it may cause at least one in six embryos with reproductive potential to be discarded and prevent miscarriage in less than one in three embryos in single-gene disease carriers.
Similar content being viewed by others
References
Verlinsky Y, Cieslak J, Freidine M, Ivakhnenko V, Wolf G, Kovalinskaya L, et al. Pregnancies following pre-conception diagnosis of common aneuploidies by fluorescent in-situ hybridization. Hum Reprod. 1995;10:1923–7. https://doi.org/10.1093/oxfordjournals.humrep.a136207.
Hardarson T, Hanson C, Lundin K, Hillensjö T, Nilsson L, Stevic J, et al. Preimplantation genetic screening in women of advanced maternal age caused a decrease in clinical pregnancy rate: a randomized controlled trial. Hum Reprod. 2008;23:2806–12. https://doi.org/10.1093/humrep/den217.
Harton GL, De Rycke M, Fiorentino F, Moutou C, SenGupta S, Traeger-Synodinos J, et al. ESHRE PGD consortium best practice guidelines for amplification-based PGD. Hum Reprod. 2011;26:33–40. https://doi.org/10.1093/humrep/deq231.
Mastenbroek S, Twisk M, van Echten-Arends J, Sikkema-Raddatz B, Korevaar JC, Verhoeve HR, et al. In vitro fertilization with preimplantation genetic screening. N Engl J Med. 2007;357:9–17. https://doi.org/10.1056/NEJMoa067744.
Practice Committee of Society for Assisted Reproductive Technology, Practice Committee of American Society for Reproductive Medicine. Preimplantation genetic testing: a Practice Committee opinion. Fertil Steril 2008;90;Suppl:S136–43. https://doi.org/10.1016/j.fertnstert.2008.08.062
Forman EJ, Hong KH, Ferry KM, Tao X, Taylor D, Levy B, et al. In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertil Steril. 2013;100:100-7.e1. https://doi.org/10.1016/j.fertnstert.2013.02.056.
Gleicher N, Barad DH. A review of, and commentary on, the ongoing second clinical introduction of preimplantation genetic screening (PGS) to routine IVF practice. J Assist Reprod Genet. 2012;29:1159–66. https://doi.org/10.1007/s10815-012-9871-2.
Scott RT Jr, Upham KM, Forman EJ, Hong KH, Scott KL, Taylor D, et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertil Steril. 2013;100:697–703. https://doi.org/10.1016/j.fertnstert.2013.04.035.
Yang Z, Liu J, Collins GS, Salem SA, Liu X, Lyle SS, et al. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Mol Cytogenet. 2012;5:24. https://doi.org/10.1186/1755-8166-5-24.
Barbash-Hazan S, Frumkin T, Malcov M, Yaron Y, Cohen T, Azem F, et al. Preimplantation aneuploid embryos undergo self-correction in correlation with their developmental potential. Fertil Steril. 2009;92:890–6. https://doi.org/10.1016/j.fertnstert.2008.07.1761.
Bolton H, Graham SJL, Van der Aa N, Kumar P, Theunis K, Fernandez Gallardo E, et al. Mouse model of chromosome mosaicism reveals lineage-specific depletion of aneuploid cells and normal developmental potential. Nat Commun. 2016;7:11165. https://doi.org/10.1038/ncomms11165.
Greco E, Minasi MG, Fiorentino F. Healthy babies after intrauterine transfer of mosaic aneuploid blastocysts. N Engl J Med. 2015;373:2089–90. https://doi.org/10.1056/NEJMc1500421.
Spinella F, Fiorentino F, Biricik A, Bono S, Ruberti A, Cotroneo E, et al. Extent of chromosomal mosaicism influences the clinical outcome of in vitro fertilization treatments. Fertil Steril. 2018;109:77–83. https://doi.org/10.1016/j.fertnstert.2017.09.025.
Johnson DS, Cinnioglu C, Ross R, Filby A, Gemelos G, Hill M, et al. Comprehensive analysis of karyotypic mosaicism between trophectoderm and inner cell mass. Mol Hum Reprod. 2010;16:944–9. https://doi.org/10.1093/molehr/gaq062.
Liu J, Wang W, Sun X, Liu L, Jin H, Li M, et al. DNA microarray reveals that high proportions of human blastocysts from women of advanced maternal age are aneuploid and mosaic. Biol Reprod. 2012;87:148. https://doi.org/10.1095/biolreprod.112.103192.
Orvieto R. Re-analysis of aneuploidy blastocysts with an inner cell mass and different regional trophectoderm cells. J Assist Reprod Genet. 2017;34:827. https://doi.org/10.1007/s10815-017-0914-6.
Popovic M, Dheedene A, Christodoulou C, Taelman J, Dhaenens L, Van Nieuwerburgh F, et al. Chromosomal mosaicism in human blastocysts: the ultimate challenge of preimplantation genetic testing? Hum Reprod. 2018;33:1342–54. https://doi.org/10.1093/humrep/dey106.
Gleicher N, Vidali A, Braverman J, Kushnir VA, Barad DH, Hudson C, et al. Accuracy of preimplantation genetic screening (PGS) is compromised by degree of mosaicism of human embryos. Reprod Biol Endocrinol. 2016;14:54. https://doi.org/10.1186/s12958-016-0193-6.
Gleicher N, Metzger J, Croft G, Kushnir VA, Albertini DF, Barad DH. A single trophectoderm biopsy at blastocyst stage is mathematically unable to determine embryo ploidy accurately enough for clinical use. Reprod Biol Endocrinol. 2017;15:33. https://doi.org/10.1186/s12958-017-0251-8.
Pinard R, de Winter A, Sarkis GJ, Gerstein MB, Tartaro KR, Plant RN, et al. Assessment of whole genome amplification-induced bias through high-throughput, massively parallel whole genome sequencing. BMC Genomics. 2006;7:216. https://doi.org/10.1186/1471-2164-7-216.
Sabina J, Leamon JH. Bias in whole genome amplification: causes and considerations. Methods Mol Biol. 2015;1347:15–41. https://doi.org/10.1007/978-1-4939-2990-0_2.
Homer HA. Preimplantation genetic testing for aneuploidy (PGT-A): the biology, the technology and the clinical outcomes. Aust N Z J Obstet Gynaecol. 2019;59:317–24. https://doi.org/10.1111/ajo.12960.
Maxwell SM, Colls P, Hodes-Wertz B, McCulloh DH, McCaffrey C, Wells D, et al. Why do euploid embryos miscarry? A case-control study comparing the rate of aneuploidy within presumed euploid embryos that resulted in miscarriage or live birth using next-generation sequencing. Fertil Steril. 2016;106(1414):1414-1419.e5. https://doi.org/10.1016/j.fertnstert.2016.08.017.
Cram DS, Leigh D, Handyside A, Rechitsky L, Xu K, Harton G, et al. PGDIS position statement on the transfer of mosaic embryos 2019. Reprod Biomed Online. 2019;39(1;Suppl 1):e1–4. https://doi.org/10.1016/j.rbmo.2019.06.012.
Chen D, Shen X, Wu C, Xu Y, Ding C, Zhang G, et al. Eleven healthy live births: a result of simultaneous preimplantation genetic testing of α- and β-double thalassemia and aneuploidy screening. J Assist Reprod Genet. 2020;37:549–57. https://doi.org/10.1007/s10815-020-01732-7.
Fu Y, Shen X, Chen D, Wang Z, Zhou C. Multiple displacement amplification as the first step can increase the diagnostic efficiency of preimplantation genetic testing for monogenic disease for β-thalassemia. J Obstet Gynaecol Res. 2019;45:1515–21. https://doi.org/10.1111/jog.14003.
Shen X, Xu Y, Zhong Y, Zhou C, Zeng Y, Zhuang G, et al. Preimplantation genetic diagnosis for α-and β-double thalassemia. J Assist Reprod Genet. 2011;28:957–64. https://doi.org/10.1007/s10815-011-9598-5.
Marin D, Zimmerman R, Tao X, Zhan Y, Scott RT Jr, Treff NR. Validation of a targeted next generation sequencing-based comprehensive chromosome screening platform for detection of triploidy in human blastocysts. Reprod Biomed Online. 2018;36:388–95. https://doi.org/10.1016/j.rbmo.2017.12.015.
Tiegs AW, Tao X, Zhan Y, Whitehead C, Kim J, Hanson B, et al. A multicenter, prospective, blinded, nonselection study evaluating the predictive value of an aneuploid diagnosis using a targeted next-generation sequencing-based preimplantation genetic testing for aneuploidy assay and impact of biopsy. Fertil Steril. 2021;115:627–37. https://doi.org/10.1016/j.fertnstert.2020.07.052.
Lin PY, Lee CI, Cheng EH, Huang CC, Lee TH, Shih HH, et al. Clinical outcomes of single mosaic embryo transfer: high-level or low-level mosaic embryo, does it matter? J Clin Med. 2020;9(6):1695. https://doi.org/10.3390/jcm9061695.
Friedenthal J, Maxwell SM, Munné S, Kramer Y, McCulloh DH, McCaffrey C, et al. Next generation sequencing for preimplantation genetic screening improves pregnancy outcomes compared with array comparative genomic hybridization in single thawed euploid embryo transfer cycles. Fertil Steril. 2018;109:627–32. https://doi.org/10.1016/j.fertnstert.2017.12.017.
Niu W, Wang L, Xu J, Li Y, Shi H, Li G, et al. Improved clinical outcomes of preimplantation genetic testing for aneuploidy using MALBAC-NGS compared with MDA-SNP array. BMC Pregnancy Childbirth. 2020;20:388. https://doi.org/10.1186/s12884-020-03082-9.
Xiao M, Lei CX, Xi YP, Lu YL, Wu JP, Li XY, et al. Next-generation sequencing is more efficient at detecting mosaic embryos and improving pregnancy outcomes than single-nucleotide polymorphism array analysis. J Mol Diagn. 2021;23:710–8. https://doi.org/10.1016/j.jmoldx.2021.02.011.
Ozgur K, Berkkanoglu M, Bulut H, Yoruk GDA, Candurmaz NN, Coetzee K. Single best euploid versus single best unknown-ploidy blastocyst frozen embryo transfers: a randomized controlled trial. J Assist Reprod Genet. 2019;36:629–36. https://doi.org/10.1007/s10815-018-01399-1.
Doyle N, Gainty M, Eubanks A, Doyle J, Hayes H, Tucker M, et al. Donor oocyte recipients do not benefit from preimplantation genetic testing for aneuploidy to improve pregnancy outcomes. Hum Reprod. 2020;35:2548–55. https://doi.org/10.1093/humrep/deaa219.
McCoy RC. Mosaicism in preimplantation human embryos: when chromosomal abnormalities are the norm. Trends Genet. 2017;33:448–63. https://doi.org/10.1016/j.tig.2017.04.001.
Deng J, Hong HY, Zhao Q, Nadgauda A, Ashrafian S, Behr B, et al. Preimplantation genetic testing for aneuploidy in poor ovarian responders with four or fewer oocytes retrieved. J Assist Reprod Genet. 2020;37:1147–54. https://doi.org/10.1007/s10815-020-01765-y.
Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98:1103–11. https://doi.org/10.1016/j.fertnstert.2012.06.048
Popescu F, Jaslow CR, Kutteh WH. Recurrent pregnancy loss evaluation combined with 24-chromosome microarray of miscarriage tissue provides a probable or definite cause of pregnancy loss in over 90% of patients. Hum Reprod. 2018;33:579–87. https://doi.org/10.1093/humrep/dey021.
Victor AR, Griffin DK, Brake AJ, Tyndall JC, Murphy AE, Lepkowsky LT, et al. Assessment of aneuploidy concordance between clinical trophectoderm biopsy and blastocyst. Hum Reprod. 2019;34:181–92. https://doi.org/10.1093/humrep/dey327.
Sachdev NM, McCulloh DH, Kramer Y, Keefe D, Grifo JA. The reproducibility of trophectoderm biopsies in euploid, aneuploid, and mosaic embryos using independently verified next-generation sequencing (NGS): a pilot study. J Assist Reprod Genet. 2020;37:559–71. https://doi.org/10.1007/s10815-020-01720-x.
Girardi L, Serdarogullari M, Patassini C, Poli M, Fabiani M, Caroselli S, et al. Incidence, origin, and predictive model for the detection and clinical management of segmental aneuploidies in human embryos. Am J Hum Genet. 2020;106:525–34. https://doi.org/10.1016/j.ajhg.2020.03.005.
Coonen E, van Montfoort A, Carvalho F, Kokkali G, Moutou C, Rubio C, et al. ESHRE PGT Consortium data collection XVI-XVIII: cycles from 2013 to 2015. Hum Reprod Open. 2020;2020:h0aa043. https://doi.org/10.1093/hropen/hoaa043.
Acknowledgements
The study was supported by the National Natural Science Foundation of China (32000589), Guangdong Basic and Applied Basic Research Foundation (2114050000636), National Key R&D Program of China (2016YFC1000205), and Guangdong Provincial Key Laboratory of Reproductive Medicine (2012A061400003). We sincerely thank all of the staff of the Reproductive Medicine Center of the First Affiliated Hospital of Sun Yat-sen University for their contributions to this study.
Author information
Authors and Affiliations
Contributions
X.T.S. and D.J.C. contributed to the interpretation of data and drafted the article. C.H.D., Y.X., Y.F., and B.C. were responsible for the analysis of data and revising the manuscript critically for important intellectual content. Y.L.W., J.W., R.L., J.G., J.F.P., H.Z., and Y.H.Z. participated in the acquisition of data. C.Q.Z. contributed to the conception and design and critical review of the article. All authors have approved the final version of the manuscript to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaoting Shen and Dongjia Chen contributed equally to this work and share first authorship.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shen, X., Chen, D., Ding, C. et al. Evaluating the application value of NGS-based PGT-A by screening cryopreserved MDA products of embryos from PGT-M cycles with known transfer outcomes. J Assist Reprod Genet 39, 1323–1331 (2022). https://doi.org/10.1007/s10815-022-02447-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02447-7