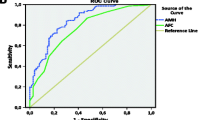
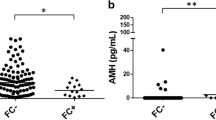
Abstract
Purpose
To evaluate the association between anti-Müllerian hormone (AMH) and follicle density in infertile women with diminished ovarian reserve (DOR) versus women with normal ovarian reserve?
Methods
Case–control study comparing follicle densities in ovarian cortex from 20 infertile women with DOR (AMH ≤ 5 pmol/L) and 100 controls with presumed normal ovarian reserve.
Results
For all women > 25 years, the follicle densities correlated positively with AMH levels. For each single picomole per liter increase in AMH the follicle density increased by 6% (95% CI 3.3–8.5%) when adjusted for age. This was similar for women with DOR and controls. The follicle density was 1.8 follicles/mm3 cortical tissue in women with DOR versus 7.0 in age-paired controls (p = 0.04). The women with DOR had a median AMH of 1.8 pmol/L versus 14.4 pmol/L in the age-paired control group (p < 0.001). The ratio of AMH/follicle density was 1:1 (1.8/1.8) in women with DOR and 2:1 (14.4/7.0) in the age-paired controls. Analyses for gonadotropin receptor polymorphisms could not explain the characteristics of women with DOR. The proportion of secondary follicles was higher in women with DOR compared with controls (4.6% versus 1.4%, p = 0.0003). Pooling all patients, the follicle density decreased significantly by 7.7% for every year added (p < 0.0001). The women with DOR had lower follicle densities than the controls, but the slopes were equal in the two cohorts.
Conclusions
Follicle density and AMH concentrations correlate also when AMH is low. However, AMH is only a reliable marker for the true ovarian reserve when age is included in the estimation and women with DOR may have more follicles than their AMH levels imply.



Similar content being viewed by others
References
Pastore LM, Christianson MS, Stelling J, Kearns WG, Segars JH. Reproductive ovarian testing and the alphabet soup of diagnoses: DOR, POI, POF, POR, and FOR. J Assist Reprod Genet. 2018;35:17–23.
Jeppesen J V., Anderson RA, Kelsey TW, Christiansen SL, Kristensen SG, Jayaprakasan K, et al. Which follicles make the most anti-Mullerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Mol Hum Reprod [Internet]. 2013 [cited 2014 May 1];19:519–27. Available from: http://molehr.oxfordjournals.org.ep.fjernadgang.kb.dk/content/19/8/519.full
Depmann M, Broer SL, Van Der Schouw YT, Tehrani FR, Eijkemans MJ, Mol BW, et al. Can we predict age at natural menopause using ovarian reserve tests or mother’s age at menopause? A systematic literature review. Menopause. 2016;23:224–32.
Depmann M, Faddy MJ, van der Schouw YT, Peeters PHM, Broer SL, Kelsey TW, et al. The relationship between variation in size of the primordial follicle pool and age at natural menopause. J Clin Endocrinol Metab [Internet]. Endocrine Society Chevy Chase, MD; 2015 [cited 2016 Jan 19];100:E845-51. Available from:http://press.endocrine.org.ep.fjernadgang.kb.dk/doi/abs/10.1210/jc.2015-1298 8
Kelsey TW, Anderson RA, Wright P, Nelson SM, Wallace WHB. Data-driven assessment of the human ovarian reserve. Mol Hum Reprod. 2012;18:79–87.
Garavaglia E, Sala C, Taccagni G, Traglia M, Barbieri C, Ferrari S, et al. Fertility preservation in endometriosis patients: anti-Müllerian hormone is a reliable marker of the ovarian follicle density. Front Surg [Internet]. 2017;4:1–6. https://doi.org/10.3389/fsurg.2017.00040/full.
Hansen KR, Hodnett GM, Knowlton N. Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril [Internet] Elsevier Ltd; 2011 [cited 2014 Apr 27];95:170–5. Available from:. https://doi.org/10.1016/j.fertnstert.2010.04.006.
Sermondade N, Sonigo C, Sifer C, Valtat S, Ziol M, Eustache F, et al. Serum antimullerian hormone is associated with the number of oocytes matured in vitro and with primordial follicle density in candidates for fertility preservation. Fertil Steril [Internet]. 2019;111:357–62 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0015028218311439.
Lunding SA, Pors SE, Kristensen SG, Landersoe SK, Jeppesen JV, Flachs EM, et al. Biopsying, fragmentation and autotransplantation of fresh ovarian cortical tissue in infertile women with diminished ovarian reserve. Hum Reprod [Internet]. 2019;34:1924–19236. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31593582
Hagen CP, Aksglaede L, Sørensen K, Main KM, Boas M, Cleemann L, et al. Serum levels of anti-Müllerian hormone as a marker of ovarian function in 926 healthy females from birth to adulthood and in 172 Turner syndrome patients J Clin Endocrinol Metab [Internet]. 2010 [cited 2014 Mar 10];95:5003–10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20719830
Kelsey TW, Dodwell SK, Wilkinson AG, Greve T, Andersen CY, Anderson RA, et al. Ovarian volume throughout life: a validated normative model. PLoS One. 2013;8.
Schmidt KLT, Byskov AG, Nyboe Andersen A, Müller J, Yding Andersen C. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum Reprod [Internet]. 2003 [cited 2016 Mar 9];18:1158–64. Available from: http://humrep.oxfordjournals.org.ep.fjernadgang.kb.dk/content/18/6/1158
Li HWR, Wong BPC, Ip WK, Yeung WSB, Ho PC, Ng EHY. Comparative evaluation of three new commercial immunoassays for anti-Müllerian hormone measurement. Hum Reprod [Internet]. 2016;31:2796–802. Available from:. https://doi.org/10.1093/humrep/dew248.
Tadros T, Tarasconi B, Nassar J, Benhaim JL, Taieb J, Fanchin R. New automated antimüllerian hormone assays are more reliable than the manual assay in patients with reduced antral follicle count. Fertil Steril. 2016;106:1800–6.
Friis Petersen J, Løkkegaard E, Andersen LF, Torp K, Egeberg A, Hedegaard L, et al. A randomized controlled trial of AMH-based individualized FSH dosing in a GnRH antagonist protocol for IVF. Hum Reprod Open. 2019;2019:1–11.
Rosendahl M, Ernst E, Rasmussen PE, Andersen CY. True ovarian volume is underestimated by two-dimensional transvaginal ultrasound measurement. Fertil Steril [Internet]. Elsevier Ltd. 2010;93:995–8. https://doi.org/10.1016/j.fertnstert.2008.10.055.
Borgbo T, Sommer Kristensen L, Lindgren I, Yding Andersen C, Hansen LL. Genotyping common FSHR polymorphisms based on competitive amplification of differentially melting amplicons (CADMA). J Assist Reprod Genet. 2014;31:1427–36.
Lindgren I, Baath M, Uvebrant K, Dejmek A, Kjaer L, Henic E, et al. Combined assessment of polymorphisms in the LHCGR and FSHR genes predict chance of pregnancy after in vitro fertilization. Hum Reprod. 2016;31:672–83.
Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7:1342–6.
Wallace WHB, Kelsey TW. Human ovarian reserve from conception to the menopause. PLoS One. 2010;5:1–9.
Bentzen JG, Forman JL, Johannsen TH, Pinborg A, Larsen EC, Andersen AN. Ovarian antral follicle subclasses and anti-Müllerian hormone during normal reproductive aging. J Clin Endocrinol Metab. 2013;98:1602–11.
Richardson MC, Guo M, Fauser BCJM, Macklon NS. Environmental and developmental origins of ovarian reserve. Hum Reprod Update. 2014;20:353–69.
te Velde E, Scheffer G, Dorland M, Broekmans F, Fauser BCJ. Developmental and endocrine aspects of normal ovarian aging. Mol Cell Endocrinol [Internet]. 1998;145:67–73 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0303720798001713.
te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update. 2002;8:141–54.
Abrahami N, Izhaki I, Younis JS. Do young women with unexplained infertility show manifestations of decreased ovarian reserve ? J Assist Reprod Genet. J Assist Reprod Genet. 2019;36:1143–52.
Ata B, Seyhan A, Seli E. Diminished ovarian reserve versus ovarian aging : overlaps and differences. Curr Opin Obstet Gynecol. 2019.
Gottschalk MS, Eskild A, Tanbo TG, Bjelland EK. Childbirth close to natural menopause: does age at menopause matter? Reprod Biomed Online [Internet]. Elsevier Ltd; 2019;39:169–75. Available from. https://doi.org/10.1016/j.rbmo.2019.03.209.
Richardson A, Mascarenhas M, Balen A. Is a woman’s chronological age or ‘ovarian age’ more important in determining perinatal outcome after assisted reproductive treatment ? Hum Fertil [Internet] Taylor & Francis; 2019;0:1–7. Available from: https://doi.org/10.1080/14647273.2019.1597987
Ernst EH, Grøndahl ML, Grund S, Hardy K, Heuck A, Sunde L, et al. Dormancy and activation of human oocytes from primordial and primary follicles: molecular clues to oocyte regulation Hum Reprod [Internet]. 2017 [cited 2018 Feb 22];32:1684–700. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28854595
Bungum L, Tagevi J, Jokubkiene L, Bungum M, Giwercman A, Macklon N, et al. The impact of the biological variability or assay performance on AMH measurements: a prospective cohort study with AMH tested on three analytical assay-platforms. Front Endocrinol (Lausanne). 2018;9:1–10.
Melado L, Lawrenz B, Sibal J, Abu E, Coughlan C, Navarro AT, et al. Anti-müllerian hormone during natural cycle presents significant intra and intercycle variations when measured with fully automated assay. Front Endocrinol (Lausanne). 2018;9:1–8.
Acknowledgments
The authors would like to thank all personnel from the clinical service in fertility preservation.
Funding
This study is part of ReproUnion collaborative study, co-financed by the European Union, Interreg V ÖKS (grant number 20200407).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The patients recruited for the clinical study [9] all signed a written informed consent in which it was specified that one biopsy would be used for histological examination. The clinical study was approved by the Scientific Ethical Committee of the Capital Region of Denmark (H-15011975) and The Danish Data Protection Agency (2012-58-0004).
The control group was recruited from the research program on cryopreservation of ovarian tissue prior to treatment of malignant disease and was approved by the Ethical Committee of Copenhagen and Frederiksberg (H-2-2001-044). Retrospective follow-up was approved by the Danish patient safety authority (3-3013-2790/1).
Disclaimer
The funders had no role in the study design, data collection and interpretation, or decision to submit the work for publication.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lunding, S.A., Pors, S.E., Kristensen, S.G. et al. Ovarian cortical follicle density in infertile women with low anti-Müllerian hormone. J Assist Reprod Genet 37, 109–117 (2020). https://doi.org/10.1007/s10815-019-01633-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-019-01633-4