Abstract
Purpose
We aimed to investigate the angiogenic balance in fresh compared to frozen embryo transfers, and among neonates with adverse perinatal outcomes.
Methods
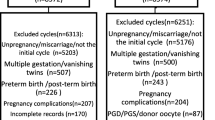
This was a retrospective cohort study. All IVF cycles resulting in a singleton live birth at a university academic fertility center from January 1, 2011, to December 31, 2013, were examined. Concentrations of sFLT-1 and PlGF were measured in previously frozen serum specimens collected during early gestation at approximately 5 weeks gestation. Patients completed an electronic survey to detail perinatal outcome.
Results
We identified 152 singleton live births (103 fresh, 49 frozen). Demographic characteristics were similar between the two groups. Ratios of sFlt-1:PlGF were not different between fresh and frozen transfers. Neonates from fresh cycles had a mean birth weight 202 g lighter (p = 0.01) than frozen cycles, after adjusting for gestational age. Among babies born with poor perinatal outcomes, there was a difference in sFlt-1:PlGF ratios after adjusting for race. In non-Asians, infants born small for gestational age (SGA) (< 10th percentile) had significantly higher sFLT-1:PLGF ratio, median ratio (0.21 vs 0.12, p = 0.016).
Conclusions
Fresh transfers were associated with lower birth weight infants compared to frozen transfers. While there was no difference in sFlt-1:PlGF ratios between fresh and frozen transfers, these ratios were significantly lower in SGA infants, suggesting an imbalance in angiogenic markers during placentation.

Similar content being viewed by others
References
Kaufmann P, Mayhew TM, Charnock-Jones DS. Aspects of human fetoplacental vasculogenesis and angiogenesis. II. Changes during normal pregnancy. Placenta. 2004;25(2–3):114–26.
Barut F, Barut A, Gun BD, et al. Intrauterine growth restriction and placental angiogenesis. Diagn Pathol. 2010;5:24.
Ahmed A, Perkins J. Angiogenesis and intrauterine growth restriction. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14(6):981–98.
Wa Law L, Sahota DS, Chan LW, Chen M, Lau TK, Leung TY. Serum placental growth factor and fms-like tyrosine kinase 1 during first trimester in Chinese women with pre-eclampsia—a case-control study. J Matern Fetal Neonatal Med. 2011;24(6):808–11.
Vatten LJ, Asvold BO, Eskild A. Angiogenic factors in maternal circulation and preeclampsia with or without fetal growth restriction. Acta Obstet Gynecol Scand. 2012;91(12):1388–94.
Anderson UD, Olsson MG, Kristensen KH, Akerstrom B, Hansson SR. Review: biochemical markers to predict preeclampsia. Placenta. 2012;33Suppl:S42–7.
Espinoza J, Uckele JE, Starr RA, Seubert DE, Espinoza AF, Berry SM. Angiogenic imbalances: the obstetric perspective. Am J Obstet Gynecol. 2010;203(1):17.e11–8.
Wender-Ozegowska E, Zawiejska A, Iciek R, Brazert J. Concentrations of eNOS, VEGF, ACE and PlGF in maternal blood as predictors of impaired fetal growth in pregnancy complicated by gestational hypertension/preeclampsia. Hypertens Pregnancy. 2015;34(1):17–23.
Sanchez O, Llurba E, Marsal G, et al. First trimester serum angiogenic/anti-angiogenic status in twin pregnancies: relationship with assisted reproduction technology. Hum Reprod. 2012;27(2):358–65.
Lee MS, Cantonwine D, Little SE, et al. Angiogenic markers in pregnancies conceived through in vitro fertilization. Am J Obstet Gynecol. 2015;213(2):212.e211–8.
Pinborg A, Loft A, Aaris Henningsen AK, Rasmussen S, Andersen AN. Infant outcome of 957 singletons born after frozen embryo replacement: the Danish National Cohort Study 1995–2006. Fertil Steril. 2010;94(4):1320–7.
Lancaster PAL. High-incidence of preterm births and early losses in pregnancy after in vitro fertilization. Br Med J. 1985;291(6503):1160–3.
Wang YA, Sullivan EA, Black D, Dean J, Bryant J, Chapman M. Preterm birth and low birth weight after assisted reproductive technology-related pregnancy in Australia between 1996 and 2000. Fertil Steril. 2005;83(6):1650–8.
Roque M, Lattes K, Serra S, et al. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: a systematic review and meta-analysis. Fertil Steril. 2013;99(1):156–62.
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011;96(2):344–8.
Aflatoonian A, Oskouian H, Ahmadi S, Oskouian L. Can fresh embryo transfers be replaced by cryopreserved-thawed embryo transfers in assisted reproductive cycles? A randomized controlled trial. J Assist Reprod Genet. 2010;27(7):357–63.
Imudia AN, Awonuga AO, Doyle JO, et al. Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and preeclampsia in singleton pregnancies after in vitro fertilization. Fertil Steril. 2012;97(6):1374–9.
Evans J, Hannan NJ, Edgell TA, et al. Fresh versus frozen embryo transfer: backing clinical decisions with scientific and clinical evidence. Hum Reprod Update. 2014;
Wennerholm UB, Henningsen AKA, Romundstad LB, et al. Perinatal outcomes of children born after frozen-thawed embryo transfer: a Nordic cohort study from the CoNARTaS group. Hum Reprod. 2013;28(9):2545–53.
Maheshwari A, Kalampokas T, Davidson J, Bhattacharya S. Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of blastocyst-stage versus cleavage-stage embryos generated through in vitro fertilization treatment: a systematic review and meta-analysis. Fertil Steril. 2013;100(6):1615–1621.
Roque M. Freeze-all policy: is it time for that?. J Assist Reprod Genet. 2015;32(2):171–6.
Chaiworapongsa T, Romero R, Whitten AE, et al. The use of angiogenic biomarkers in maternal blood to identify which SGA fetuses will require a preterm delivery and mothers who will develop pre-eclampsia. J Matern Fetal Neonatal Med. 2015:1–15.
Lobmaier SM, Figueras F, Mercade I, et al. Angiogenic factors vs Doppler surveillance in the prediction of adverse outcome among late-pregnancy small-for-gestational-age fetuses. Ultrasound Obstet Gynecol. 2014;43(5):533–40.
Birdir C, Fryze J, Frolich S, et al. Impact of maternal serum levels of Visfatin, AFP, PAPP-A, sFlt-1 and PlGF at 11–13 weeks gestation on small for gestational age births. J Matern Fetal Neonatal Med. 2017;30(6):629–34.
Sung KU, Roh JA, Eoh KJ, Kim EH. Maternal serum placental growth factor and pregnancy-associated plasma protein A measured in the first trimester as parameters of subsequent pre-eclampsia and small-for-gestational-age infants: a prospective observational study. Obstet Gynecol Sci. 2017;60(2):154–62.
Karumanchi SA, Bdolah Y. Hypoxia and sFlt-1 in preeclampsia: the “chicken-and-egg” question. Endocrinology. 2004;145(11):4835–7.
Acknowledgements
The authors gratefully acknowledge the use of the technical support and laboratory facilities of the University of Southern California Reproductive Endocrine Clinical Laboratory at the Keck School of Medicine, University of Southern California, funded by the USC Department of Obstetrics and Gynecology seed funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests and have not received financial support for this work.
Rights and permissions
About this article
Cite this article
Woo, I., Chan, Y., Sriprasert, I. et al. The role of angiogenic markers in adverse perinatal outcomes: fresh versus frozen embryo transfers. J Assist Reprod Genet 34, 1639–1643 (2017). https://doi.org/10.1007/s10815-017-1023-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-017-1023-2