Abstract
Purpose
Artificial oocyte activation using calcium ionophores and enhancement of embryonic developmental potential by the granulocyte-macrophage colony-stimulating factor (GM-CSF) have already been reported. In this study, we evaluated the synergistic effect of these two methods on aged human unfertilized oocytes after intracytoplasmic sperm injection (ICSI). Then, we cultured the resulting embryos to the blastocyst stage and screened them for chromosomal abnormalities, to assess the safety of this protocol.
Methods
Aged human oocytes deemed unfertilized after ICSI were activated, either by briefly applying the calcium ionophore A23187 alone (group A) or by briefly applying the ionophore and then supplementing the culture medium with recombinant human GM-CSF (rhGM-CSF) (group B). Next, the development was monitored in a time-lapse incubator system, and ploidy was analyzed by array comparative genomic hybridization (aCGH), after whole embryo biopsy and whole genome amplification. Differences between oocytes and resulting embryos in both groups were evaluated statistically.
Results
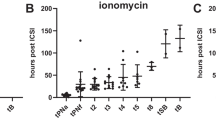
Oocytes unfertilized after ICSI can be activated with the calcium ionophore A23187 to show two pronuclei and two polar bodies. Addition of rhGM-CSF in the culture medium of A23187-activated oocytes enhances their cleaving and blastulation potential and results in more euploid blastocysts compared to the culture medium alone.
Conclusions
This study shows that activating post-ICSI aged human unfertilized oocytes with a combination of a calcium ionophore and a cytokine can produce good-morphology euploid blastocysts.


Similar content being viewed by others
References
Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–8.
Liu J, Nagy Z, Joris H, Tournaye H, Smitz J, Camus M, et al. Analysis of 76 total fertilization failure cycles out of 2732 intracytoplasmic sperm injection cycles. Hum Reprod. 1995;10:2630–6.
Moomjy M, Sills ES, Rosenwaks Z, Palermo GD. Implications of complete fertilization failure after intracytoplasmic sperm injection for subsequent fertilization and reproductive outcome. Hum Reprod. 1998;13:2212–6.
de Klerk C, Macklon NS, Heijnen EM, Eijkemans MJ, Fauser BC, Passchier J, et al. The psychological impact of IVF failure after two or more cycles of IVF with a mild versus standard treatment strategy. Hum Reprod. 2007;22:2554–8.
Tesarik J, Rienzi L, Ubaldi F, Mendoza C, Greco E. Use of a modified intracytoplasmic sperm injection technique to overcome sperm-borne and oocyte-borne oocyte activation failures. Fertil Steril. 2002;78:619–24.
Rawe VY, Kopelman S, Nodar FN, Olmedo SB, Chillik CF. Pronuclear abnormalities and cytoskeletal organization during assisted fertilization in a patient with multifollicular ovarian response. J Assist Reprod Genet. 2002;19:152–7.
Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, et al. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development. 2002;129:3533–44.
Ebner T, Oppelt P, Wober M, Staples P, Mayer RB, Sonnleitner U, et al. Treatment with Ca2+ ionophore improves embryo development and outcome in cases with previous developmental problems: a prospective multicenter study. Hum Reprod. 2015;30:97–102.
Nomikos M. Novel signalling mechanism and clinical applications of sperm-specific PLCζ. Biochem Soc Trans. 2015;43:371–6.
Kashir J, Nomikos M, Swann K, Lai FA. PLCζ or PAWP: revisiting the putative mammalian sperm factor that triggers egg activation and embryogenesis. Mol Hum Reprod. 2015;21:383–8.
Nikiforaki D, Vanden Meerschaut F, de Roo C, Lu Y, Ferrer-Buitrago M, de Sutter P, et al. Effect of two assisted oocyte activation protocols used to overcome fertilization failure on the activation potential and calcium releasing pattern. Fertil Steril. 2016;105:798–806.
Baltaci V, Ayvaz OU, Unsal E, Aktas Y, Baltaci A, Turhan F, et al. The effectiveness of intracytoplasmic sperm injection combined with piezoelectric stimulation in infertile couples with total fertilization failure. Fertil Steril. 2010;94:900–4.
Mansour R, Fahmy I, Tawab NA, Kamal A, El-Demery Y, Aboulghar M, et al. Electrical activation of oocytes after intracytoplasmic sperm injection: a controlled randomized study. Fertil Steril. 2009;91:133–9.
Yanagida K, Katayose H, Yazawa H, Kimura Y, Sato A, Yanagimachi H, et al. Successful fertilization and pregnancy following ICSI and electrical oocyte activation. Hum Reprod. 1999;14:1307–11.
Ebner T, Moser M, Sommergruber M, Jesacher K, Tews G. Complete oocyte activation failure after ICSI can be overcome by a modified injection technique. Hum Reprod. 2004;19:1837–41.
Liu Y, Han XJ, Liu MH, Wang SY, Jia CW, Yu L, et al. Three-day-old human unfertilized oocytes after in vitro fertilization/intracytoplasmic sperm injection can be activated by calcium ionophore a23187 or strontium chloride and develop to blastocysts. Cell Reprogram. 2014;16:276–80.
Lu Q, Zhao Y, Gao X, Li Y, Ma S, Mullen S, et al. Combination of calcium ionophore A23187 with puromycin salvages human unfertilized oocytes after ICSI. Eur J Obstet Gynecol Reprod Biol. 2006;126:72–6.
Ebner T, Koster M, Shebl O, Moser M, Van der Ven H, Tews G, et al. Application of a ready-to-use calcium ionophore increases rates of fertilization and pregnancy in severe male factor infertility. Fertil Steril. 2012;98:1432–7.
Ebner T, Maurer M, Oppelt P, Mayer RB, Duba HC, Costamoling W, et al. Healthy twin live-birth after ionophore treatment in a case of theophylline-resistant Kartagener syndrome. J Assist Reprod Genet. 2015;32:873–7.
Ebner T, Montag M, Oocyte Activation Study Group, Montag M, Van der Ven K, Van der Ven H, et al. Live birth after artificial oocyte activation using a ready-to-use ionophore: a prospective multicentre study. Reprod Biomed Online. 2015;30:359–65.
Eldar-Geva T, Brooks B, Margalioth EJ, Zylber-Haran E, Gal M, Silber SJ. Successful pregnancy and delivery after calcium ionophore oocyte activation in a normozoospermic patient with previous repeated failed fertilization after intracytoplasmic sperm injection. Fertil Steril. 2003;79s3:1656–8.
Lu Q, Chen X, Li Y, Zhang XH, Liang R, Zhao YP, et al. A live birth of activated one-day-old unfertilized oocyte for a patient who experienced repeatedly near-total fertilization failure after intracytoplasmic sperm injection. Chin Med J. 2012;125:546–8.
Vanden Meerschaut F, Nikiforaki D, De Gheselle S, Dullaerts V, Van den Abbeel E, Gerris J, et al. Assisted oocyte activation is not beneficial for all patients with a suspected oocyte-related activation deficiency. Hum Reprod. 2012;27:1977–84.
Check JH, Summers-Chase D, Cohen R, Brasile D. Artificial oocyte activation with calcium ionophore allowed fertilization and pregnancy in a couple with long-term unexplained infertility where the female partner had diminished EGG reserve and failure to fertilize oocytes despite intracytoplasmic sperm injection. Clin Exp Obstet Gynecol. 2010;37:263–5.
Caglar Aytac P, Kilicdag EB, Haydardedeoglu B, Simsek E, Cok T, Parlakgumus HA. Can calcium ionophore “use” in patients with diminished ovarian reserve increase fertilization and pregnancy rates? A randomized, controlled study. Fertil Steril. 2015;104:1168–74.
Borges Jr E, de Almeida Ferreira Braga DP, de Sousa Bonetti TC, Iaconelli Jr A, Franco Jr JG. Artificial oocyte activation with calcium ionophore A23187 in intracytoplasmic sperm injection cycles using surgically retrieved spermatozoa. Fertil Steril. 2009;92:131–6.
Kang HJ, Lee SH, Park YS, Lim CK, Ko DS, Yang KM, et al. Artificial oocyte activation in intracytoplasmic sperm injection cycles using testicular sperm in human in vitro fertilization. Clin Exp Reprod Med. 2015;42:45–50.
Nasr-Esfahani MH, Razavi S, Javdan Z, Tavalaee M. Artificial oocyte activation in severe teratozoospermia undergoing intracytoplasmic sperm injection. Fertil Steril. 2008;90:2231–7.
Darwish E, Magdi Y. A preliminary report of successful cleavage after calcium ionophore activation at ICSI in cases with previous arrest at the pronuclear stage. Reprod Biomed Online. 2015;31:799–804.
Ruef C, Coleman DL. Granulocyte-macrophage colony-stimulating factor: pleiotropic cytokine with potential clinical usefulness. Rev Infect Dis. 1990;12:41–62.
Giacomini G, Tabibzadeh SS, Satyaswaroop PG, Bonsi L, Vitale L, Bagnara GP, et al. Epithelial cells are the major source of biologically active granulocyte macrophage colony-stimulating factor in human endometrium. Hum Reprod. 1995;10:3259–63.
Zhao Y, Chegini N. The expression of granulocyte macrophage-colony stimulating factor (GM-CSF) and receptors in human endometrium. Am J Reprod Immunol. 1999;42:303–11.
Berkowitz RS, Faris HM, Hill JA, Anderson DJ. Localization of leukocytes and cytokines in chorionic villi of normal placentas and complete hydatidiform moles. Gynecol Oncol. 1990;37:396–400.
Uzumaki H, Okabe T, Sasaki N, Hagiwara K, Takaku F, Tobita M, et al. Identification and characterization of receptors for granulocyte colony-stimulating factor on human placenta and trophoblastic cells. Proc Natl Acad Sci U S A. 1989;86:9323–6.
Zhao Y, Rong H, Chegini N. Expression and selective cellular localization of granulocyte-macrophage colony-stimulating factor (GM-CSF) and GM-CSF alpha and beta receptor messenger ribonucleic acid and protein in human ovarian tissue. Biol Reprod. 1995;53:923–30.
Jasper MJ, Brannstrom M, Olofsson JI, Petrucco OM, Mason H, Robertson SA, et al. Granulocyte-macrophage colony-stimulating factor: presence in human follicular fluid, protein secretion and mRNA expression by ovarian cells. Mol Hum Reprod. 1996;2:555–62.
Sjoblom C, Wikland M, Robertson SA. Granulocyte-macrophage colony-stimulating factor (GM-CSF) acts independently of the beta common subunit of the GM-CSF receptor to prevent inner cell mass apoptosis in human embryos. Biol Reprod. 2002;67:1817–23.
Agerholm I, Loft A, Hald F, Lemmen JG, Munding B, Sorensen PD, et al. Culture of human oocytes with granulocyte-macrophage colony-stimulating factor has no effect on embryonic chromosomal constitution. Reprod Biomed Online. 2010;20:477–84.
Sjoblom C, Roberts CT, Wikland M, Robertson SA. Granulocyte-macrophage colony-stimulating factor alleviates adverse consequences of embryo culture on fetal growth trajectory and placental morphogenesis. Endocrinology. 2005;146:2142–53.
Sjoblom C, Wikland M, Robertson SA. Granulocyte-macrophage colony-stimulating factor promotes human blastocyst development in vitro. Hum Reprod. 1999;14:3069–76.
Robertson SA. GM-CSF regulation of embryo development and pregnancy. Cytokine Growth Factor Rev. 2007;18:287–98.
Tevkin S, Lokshin V, Shishimorova M, Polumiskov V. The frequency of clinical pregnancy and implantation rate after cultivation of embryos in a medium with granulocyte macrophage colony-stimulating factor (GM-CSF) in patients with preceding failed attempts of ART. Gynecol Endocrinol. 2014;30s1:9–12.
Ziebe S, Loft A, Povlsen BB, Erb K, Agerholm I, Aasted M, et al. A randomized clinical trial to evaluate the effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) in embryo culture medium for in vitro fertilization. Fertil Steril. 2013;99:1600–9.
Capalbo A, Ottolini CS, Griffin DK, Ubaldi FM, Handyside AH, Rienzi L. Artificial oocyte activation with calcium ionophore does not cause a widespread increase in chromosome segregation errors in the second meiotic division of the oocyte. Fertil Steril. 2016;105:807–14.
Gardner DK, Schoolcraft WB. In-vitro culture of human blastocysts. In: Jansen R, Mortimer D, editors. Towards reproductive certainty: infertility and genetics beyond 1999. Carnforth: Parthenon; 1999. p. 378–88.
Christopikou D, Tsorva E, Economou K, Shelley P, Davies S, Mastrominas M, et al. Polar body analysis by array comparative genomic hybridization accurately predicts aneuploidies of maternal meiotic origin in cleavage stage embryos of women of advanced maternal age. Hum Reprod. 2013;28:1426–34.
Tesarik J, Sousa M. More than 90% fertilization rates after intracytoplasmic sperm injection and artificial induction of oocyte activation with calcium ionophore. Fertil Steril. 1995;63:343–9.
Chi HJ, Koo JJ, Song SJ, Lee JY, Chang SS. Successful fertilization and pregnancy after intracytoplasmic sperm injection and oocyte activation with calcium ionophore in a normozoospermic patient with extremely low fertilization rates in intracytoplasmic sperm injection cycles. Fertil Steril. 2004;82:475–7.
Rybouchkin AV, Van der Straeten F, Quatacker J, De Sutter P, Dhont M. Fertilization and pregnancy after assisted oocyte activation and intracytoplasmic sperm injection in a case of round-headed sperm associated with deficient oocyte activation capacity. Fertil Steril. 1997;68:1144–7.
van Blerkom J, Cohen J, Johnson M. A plea for caution and more research in the ‘experimental’ use of ionophores in ICSI. Reprod Biomed Online. 2015;30:323–4.
Thompson P. HFEA response to ‘A plea for caution and more research in the “experimental” use of ionophores in ICSI’. Reprod Biomed Online. 2015;31:829–30.
Aghajanpour S, Ghaedi K, Salamian A, Deemeh MR, Tavalaee M, Moshtaghian J, et al. Quantitative expression of phospholipase C zeta, as an index to assess fertilization potential of a semen sample. Hum Reprod. 2011;26:2950–6.
Heindryckx B, Van der Elst J, De Sutter P, Dhont M. Treatment option for sperm- or oocyte-related fertilization failure: assisted oocyte activation following diagnostic heterologous ICSI. Hum Reprod. 2005;20:2237–41.
Vanden Meerschaut F, D’Haeseleer E, Gysels H, Thienpont Y, Dewitte G, Heindryckx B, et al. Neonatal and neurodevelopmental outcome of children aged 3–10 years born following assisted oocyte activation. Reprod Biomed Online. 2014;28:54–63.
Miller N, Biron-Shental T, Sukenik-Halevy R, Klement AH, Sharony R, Berkovitz A. Oocyte activation by calcium ionophore and congenital birth defects: a retrospective cohort study. Fertil Steril. 2016;106:590–6.
Trounson AO. The derivation and potential use of human embryonic stem cells. Reprod Fertil Dev. 2001;13:523–32.
Katz-Jaffe MG, Trounson AO, Cram DS. Mitotic errors in chromosome 21 of human preimplantation embryos are associated with non-viability. Mol Hum Reprod. 2004;10:143–7.
Katz-Jaffe MG, Trounson AO, Cram DS. Chromosome 21 mosaic human preimplantation embryos predominantly arise from diploid conceptions. Fertil Steril. 2005;84:634–43.
Mantikou E, Wong KM, Repping S, Mastenbroek S. Molecular origin of mitotic aneuploidies in preimplantation embryos. Biochim Biophys Acta. 2012;1822:1921–30.
Hardy K, Winston RM, Handyside AH. Binucleate blastomeres in preimplantation human embryos in vitro: failure of cytokinesis during early cleavage. J Reprod Fertil. 1993;98:549–58.
Harrison RH, Kuo HC, Scriven PN, Handyside AH, Ogilvie CM. Lack of cell cycle checkpoints in human cleavage stage embryos revealed by a clonal pattern of chromosomal mosaicism analysed by sequential multicolour FISH. Zygote. 2000;8:217–24.
Ruangvutilert P, Delhanty JD, Serhal P, Simopoulou M, Rodeck CH, Harper JC. FISH analysis on day 5 post-insemination of human arrested and blastocyst stage embryos. Prenat Diagn. 2000;20:552–60.
Bielanska M, Tan SL, Ao A. High rate of mixoploidy among human blastocysts cultured in vitro. Fertil Steril. 2002;78:1248–53.
Clouston HJ, Herbert M, Fenwick J, Murdoch AP, Wolstenholme J. Cytogenetic analysis of human blastocysts. Prenat Diagn. 2002;22:1143–52.
Chatzimeletiou K, Morrison EE, Prapas N, Prapas Y, Handyside AH. Spindle abnormalities in normally developing and arrested human preimplantation embryos in vitro identified by confocal laser scanning microscopy. Hum Reprod. 2005;20:672–82.
Lutz EE. Preimplantation genetic diagnosis (PGD) according to medical ethics and medical law. J Turk Ger Gynecol Assoc. 2012;13:50–5.
Rubio I, Kuhlmann R, Agerholm I, Kirk J, Herrero J, Escribá MJ, et al. Limited implantation success of direct-cleaved human zygotes: a time-lapse study. Fertil Steril. 2012;98:1458–63.
Acknowledgments
The authors wish to thank Mr. Christos Karamalegos for the help with statistical analysis and Ms. Maria Papadopoulou for collecting the patient consent forms.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
The study was approved by the institutional research ethics committee, and then it was submitted to and obtained authorization by the Hellenic National Authority of Medically Assisted Reproduction (authorization ref. 134/15), as required by national legislation for research use of human embryos not destined to embryo transfer.
Informed consent
All patients who donated oocytes and embryos to be included in the study signed consent forms, after receiving exhaustive information on the purpose of the research carried out, on the procedures to be used, on the possibility to withdraw their consent at any time up to the first actual use of each oocyte or embryo in an experiment, and on the legal and ethical obligation of the authors to destroy all embryos after completion of the experiments, without attempting to transfer them in vivo.
Additional information
Capsule The exposure of human unfertilized oocytes 18 h after ICSI to a combination of calcium ionophore A23187 and the GM-CSF cytokine can safely salvage the oocytes, resulting in the development of good-morphology, euploid blastocysts, with potential clinical use.
Rights and permissions
About this article
Cite this article
Economou, K.A., Christopikou, D., Tsorva, E. et al. The combination of calcium ionophore A23187 and GM-CSF can safely salvage aged human unfertilized oocytes after ICSI. J Assist Reprod Genet 34, 33–41 (2017). https://doi.org/10.1007/s10815-016-0823-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-016-0823-0