Abstract
Purpose
The change of mitochondrial distribution in human oocytes during meiotic maturation was assessed using 223 human oocytes donated from patients undergoing fertility treatment between June 2013 and February 2016.
Methods
Live cell images of fluorescence-labelled mitochondria in human oocytes were analysed to investigate dynamic changes in mitochondrial distribution during meiotic maturation using a confocal microscope combined with an incubator in the presence or absence of colchicine and cytochalasin B, inhibitors for tubulin and actin filament, respectively. Subcellular distribution of mitochondria in human oocytes was also assessed at various stages using a transmission electron microscope (TEM).
Results
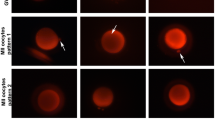
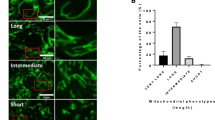
Live cell imaging analysis revealed that the mitochondria-occupied cytoplasmic area decreased from 83 to 77 % of the total cytoplasmic area around 6 h before germinal vesicle breakdown (GVBD) and that mitochondria accumulated preferentially close to the perinuclear region. Then, the mitochondria-distributed area rapidly increased to 85 % of total cytoplasm at the time of GVBD. On the other hand, there was no significant change in mitochondrial distribution before and after polar body extrusion. Such changes in mitochondrial localization were affected differently by colchicine and cytochalasin B. Most of mitochondria in the cytoplasm formed cluster-like aggregates before GVBD while they distributed homogeneously after GVBD.
Conclusions
Most mitochondria localized predominantly in the non-cortical region of the cytoplasm of GV stage-oocytes, while the mitochondria-occupied area decreased transiently before GVBD and increased rapidly to occupy the entire area of the cytoplasm at GVBD by some cytoskeleton-dependent mechanism.






Similar content being viewed by others
References
Motta PM, Nottola SA, Familiari G, Makabe S, Stallone T, Macchiarelli G. Morphodynamics of the follicular-luteal complex during early ovarian development and reproductive life. Int Rev Cytol. 2003;223:177–288.
Sathananthan AH. Ultrastructural changes during meiotic maturation in mammalian oocytes: unique aspects of the human oocyte. Microsc Res Tech. 1994;27:145–64.
Krisher RL, Bavister BD. Responses of oocytes and embryos to the culture environment. Theriogenology. 1998;59:103–14.
Van Blerkom J, Davis P, Lee J. ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum Reprod. 1995;10:415–24.
Van Blerkom J. Mitochondria in human oogenesis and preimplantation embryogenesis: engines of metabolism, ionic regulation and developmental competence. Reproduction. 2004;128:269–80.
Stojkovic M, Machado SA, Stojkovic P, Zakhartchenko V, Hutzler P, Goncalves PB, et al. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol Reprod. 2001;64:904–9.
Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11:797–813.
Dalton CM, Szabadkai G, Carroll J. Measurement of ATP in single oocytes: impact of maturation and cumulus cells on levels and consumption. J Cell Physiol. 2014;229:353–61.
Van Blerkom J. Microtubule mediation of cytoplasmic and nuclear maturation during the early stages of resumed meiosis in cultured mouse oocytes. Proc Natl Acad Sci U S A. 1991;88:5031–5.
Nagai S, Mabuchi T, Hirata S, Shoda T, Kasai T, Yokota S, et al. Correlation of abnormal mitochondrial distribution in mouse oocytes with reduced developmental competence. Tohoku J Exp Med. 2006;210:137–44.
Eichenlaub-Ritter U, Wieczorek M, Lüke S, Seidel T. Age related changes in mitochondrial function and new approaches to study redox regulation in mammalian oocytes in response to age or maturation conditions. Mitochondrion. 2011;11:783–96.
Barnett DK, Kimura J, Bavister BD. Translocation of active mitochondria during hamster preimplantation embryo development studied by confocal laser scanning microscopy. Dev Dyn. 1996;205:64–72.
Van Blerkom J, Davis P, Alexander S. Differential mitochondrial distribution in human pronuclear embryos leads to disproportionate inheritance between blastomeres: Relationship to microtubular organization, ATP content and competence. Hum Reprod. 2000;15:2621–33.
Van Blerkom J, Davis P, Mathwig V, Alexander S. Domains of high-polarized and low-polarized mitochondria may occur in mouse and human oocytes and early embryos. Hum Reprod. 2002;17:393–406.
Sun QY, Wu GM, Lai L, Park KW, Cabot R, Cheong HT, et al. Translocation of active mitochondria during pig oocyte maturation, fertilization and early embryo development in vitro. Reproduction. 2001;122:155–63.
Wilding M, Dale B, Marino M, di Matteo L, Alviggi C, Pisaturo ML, et al. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod. 2001;16:909–17.
Zhang YZ, Ouyang YC, Hou Y, Schatten H, Chen DY, Sun QY. Mitochondrial behavior during oogenesis in zebrafish: a confocal microscopy analysis. Develop Growth Differ. 2008;50:189–201.
Dumollard R, Marangos P, Fitzharris G, Swann K, Duchen M, Carroll J. Sperm-triggered [Ca2+] oscillations and Ca2+ homeostasis in the mouse egg have an absolute requirement for mitochondrial ATP production. Development. 2004;131:3057–67.
Dumollard R, Carroll J, Duchen MR, Campbell K, Swann K. Mitochondrial function and redox state in mammalian embryos. Semin Cell Dev Biol. 2009;20:346–53.
Bianchi S, Macchiarelli G, Micara G, Linari A, Boninsegna C, Aragona C, et al. Ultrastructural markers of quality are impaired in human metaphase II aged oocytes: a comparison between reproductive and in vitro aging. J Assist Reprod Genet. 2015;32:1343–58.
Van Blerkom J, Runner MN. Mitochondrial reorganization during resumption of arrested meiosis in the mouse oocyte. Am J Anat. 1984;171:335–55.
Dumollard R, Duchen M, Sardet C. Calcium signals and mitochondria at fertilisation. Semin Cell Dev Biol. 2006;17:314–23.
Liu S, Li Y, Feng HL, Yan JH, Li M, Ma SY, et al. Dynamic modulation of cytoskeleton during in vitro maturation in human oocytes. Am J Obstet Gynecol. 2010;203:e1–7.
Heggeness MH, Simon M, Singer SJ. Association of mitochondria with microtubules in cultured cells. Proc Natl Acad Sci U S A. 1978;75:3863–6.
Nangaku M, Sato-Yoshitake R, Okada Y, Noda Y, Takemura R, Yamazaki H, et al. KIF1B, a novel microtubule plus end-directed monomeric motor protein for transport of mitochondria. Cell. 1994;79:1209–20.
Azoury J, Verlhac MH, Dumont J. Actin filaments: key players in the control of asymmetric divisions in mouse oocytes. Biol Cell. 2009;101:69–76.
Yu Y, Dumollard R, Rossbach A, Lai FA, Swann K. Redistribution of mitochondria leads to bursts of ATP production during spontaneous mouse oocyte maturation. J Cell Physiol. 2010;224:672–80.
Yamochi T, Hashimoto S, Amo A, Goto H, Yamanaka M, Inoue M, et al. Mitochondrial dynamics and their intracellular traffic in porcine oocytes. Zygote. 2016. doi:10.1017/S0967199415000489.
Hashimoto S, Nakano T, Yamagata K, Inoue M, Morimoto Y, Nakaoka Y. Multinucleation per se is not always sufficient as a marker of abnormality to decide against transferring human embryos. Fertil Steril. 2016. doi:10.1016/j.fertnstert.2016.03.025.
Hashimoto S, Fukuda A, Murata Y, Kikkawa M, Oku H, Kanaya H, et al. Effect of aspiration vacuum on the developmental competence of immature human oocytes retrieved using a 20-gauge needle. Reprod Biomed Online. 2007;14:444–9.
Wang E, Babbey M, Dunn KW. Performance comparison between the high-speed Yokogawa spinning disc confocal system and single-point scanning confocal systems. J Microscopy. 2005;218:148–59.
Hashimoto S, Suzuki N, Yamanaka M, Hosoi Y, Ishizuka B, Morimoto Y. Effects of vitrification solutions and equilibration times on the morphology of cynomolgus ovarian tissues. Reprod Biomed Online. 2010;21:501–9.
Hatefi Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu Rev Biochem. 1985;54:1015–69.
McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16:R551–60.
Sweet S, Singh G. Changes in mitochondrial mass, membrane potential, and cellular adenosine triphosphate content during the cell cycle of human leukemic (HL-60) cells. J Cell Physiol. 1999;180:91–6.
De Santis L, Gandolfi F, Pennarossa G, Maffei S, Gismano E, Intra G, et al. Expression and intracytoplasmic distribution of staufen and calreticulin in maturing human oocytes. J Assist Reprod Genet. 2015;32:645–52.
Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–38.
Sánchez F, Romero S, De Vos M, Verheyen G, Smitz J. Human cumulus-enclosed germinal vesicle oocytes from early antral follicles reveal heterogeneous cellular and molecular features associated with in vitro maturation capacity. Hum Reprod. 2015;30:1396–409.
Coticchio G, Dal Canto M, Renzini MM, Guglielmo MG, Brambillasca F, Turchi D, et al. Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum Reprod Update. 2015;21:427–54.
Acknowledgments
We would like to thank Editage (www.editage.jp) for the English language editing.
Funding
Part of this work was supported by a grant from IVF Namba Clinic to S.H.
Author’s roles
Y.T., S.H., and T.Y. were involved in the literature review, experimental design, data acquisition, interpretation, and analysis and manuscript preparation. H.G., M.Y., A.A., and H.M. performed the TEM. K.I., Y.N., and Y.M. prepared the oocytes. H.M., M.I., and N.S. were involved in the manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Additional information
Capsule The mitochondria-occupied area decreased transiently before GVBD and increased rapidly to occupy the entire area of the cytoplasm at GVBD by some cytoskeleton-dependent mechanism.
Rights and permissions
About this article
Cite this article
Takahashi, Y., Hashimoto, S., Yamochi, T. et al. Dynamic changes in mitochondrial distribution in human oocytes during meiotic maturation. J Assist Reprod Genet 33, 929–938 (2016). https://doi.org/10.1007/s10815-016-0716-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-016-0716-2