Abstract
Purpose
Genetic variation may influence women’s response to ovarian stimulation therapy. The purpose of this study was to investigate any effects of genetic variants in the anti-Müllerian hormone (AMH) and AMH type II receptor genes on ovarian response/treatment outcomes and on current markers of ovarian reserve in individuals undergoing in vitro fertilisation (IVF) treatment.
Methods
In this prospective observational study, we genotyped the AMH c.146G>T, p.(Ile49Ser) and AMHR2 -482A>G variants in 603 unrelated women undergoing their first cycle of controlled ovarian stimulation for IVF and ICSI (intracytoplasmic sperm injection) using gonadotrophins at a tertiary referral centre for reproductive medicine. Pelvic ultrasound and blood hormone levels were taken on days 2–3 of the cycle. Genotypes were determined using TaqMan allelic discrimination assay. Regression analysis was performed to assess the relationship between the genotypes and the ovarian reserve markers (FSH, AMH, antral follicle count) and the early outcomes of response (number of oocytes retrieved and gonadotropin dose) as well as the treatment outcome (live birth).
Results
There were no significant associations between the variants AMH c.146G>T and AMHR2 -482A>G with ovarian response in terms of number of oocytes retrieved (p = 0.08 and p = 0.64, respectively), live births (p = 0.28 and p = 0.52) and/or markers of ovarian reserve.
Conclusions
Genotyping of the AMH c.146G>T and AMHR2 -482A>G polymorphisms does not provide additional useful information as a predictor of ovarian reserve or ovarian response and treatment outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian response to stimulation with exogenous gonadotrophins during assisted reproductive techniques (ART) can range from an inadequate response, which may result in cycle cancellation, to a high response, which can put women at risk of ovarian hyperstimulation syndrome (OHSS).
Markers of ovarian reserve, such as basal serum FSH (follicle stimulating hormone) levels, estradiol, inhibin B, anti-Müllerian hormone (AMH) levels and antral follicle count (AFC), are currently used in clinical practice to predict response to stimulation [1]. Of these markers, AMH has the advantage of having relatively stable levels throughout the menstrual cycle [2, 3] and is more accurate in providing information on the expected ovarian response to controlled stimulation [3, 4]. However, it is not highly predictive of extremes of responses, and its value has been questioned [5].
AMH is a member of the transforming growth factor beta superfamily and is produced by the granulosa cells of pre-antral and small antral follicles in the ovary. It is highly expressed in the developing follicles, but its expression diminishes at the stage for selection of the dominant follicle by FSH [6]. It is used in clinical practice as a biomarker of ovarian reserve [6]. AMH is expressed in granulosa cells and may regulate FSH sensitivity in the ovary [7]. Therefore, it has been proposed to have a role in influencing ovarian response to stimulation [6]. It exerts its biological effects through the receptor AMHR2, which is present on granulosa and theca cells.
Considering the important role of the AMH signalling pathway in regulating FSH sensitivity in the ovary and follicular recruitment and selection, it is appropriate to consider that variation in the genes encoding key proteins in the pathway may influence ovarian response. Two polymorphisms, in AMH c.146G>T, p.Ile49Ser (rs10407022) and AMH type II receptor (AMHR2) -482A>G (rs2002555) genes, have been genotyped in a number of studies. These two polymorphisms have been associated with increased follicular phase oestradiol levels [7], menopausal age related to parity [7–9], age at natural menopause [10], and severity of the polycystic ovary syndrome (PCOS), in terms of follicle number and androgen levels [11], and unexplained infertility [12]. Three previous studies have considered these polymorphisms in relation to their direct effects during ovarian stimulation in ART treatment. A study of 191 women did not find a statistically significant association between these polymorphisms and response to ovarian stimulation [13]. In the second study of 151 women by Karagiorga et al., significant differences between certain subgroups following controlled ovarian stimulation were found amongst women wild type for the AMH polymorphism, in those with more than two previous IVF attempts, basal serum FSH levels were lower (p = 0.012), and in those with lower peak serum E2 levels, fertilisation rates were higher (p = 0.037) [14]. In addition, women wild type for the AMHR2 polymorphism with more than two previous IVF attempts had a higher number of follicles, and women with higher peak serum E2 levels required a lower gonadotropin dose [14]. The third study of 186 infertile women by Peluso et al. showed an association between AMHR2 polymorphisms and FSH, estradiol and AMH levels, whilst AMH polymorphisms were associated with the number of embryos produced [15].
Given the differences in the previous reports, we undertook a study in a previously collected and characterised cohort of patients [15]. We genotyped AMH c.146G>T, p.(Ile49Ser) and AMHR2 -482A>G in 603 unrelated women who underwent ovarian stimulation using gonadotrophins and correlated genotypes with ovarian reserve markers and treatment outcomes.
Materials and methods
Subjects and assays
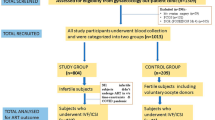
A total of 603 women undergoing assessment prior to their first IVF treatment cycle were recruited to the study. Inclusion criteria for the study were as follows: [1] presence of both ovaries [2], <40 years of age [3], no use of hormone therapy in the 6 months preceding the recruitment [4], no previous ovarian surgery or pelvic radiation therapy [5], commencing first cycle IVF treatment. In addition, the women had a body mass index (BMI) >19 and <30 kg/m2 to satisfy eligibility criteria for government-funded IVF treatment in Greater Manchester, UK [16]. The protocol was approved by the South Manchester Research Ethics Committee (REC ref no. 08/81003/212). Written informed consent was obtained from all participants.
Baseline evaluation
On days 2 to 3 of a spontaneous menstrual cycle or after a withdrawal bleed in anovulatory women, blood samples for measurement of FSH and AMH levels were obtained by venepuncture.
The FSH concentration was measured using specific immunoassay kits (Cobas; Roche Diagnostics). The intra-assay and inter-assay coefficients of variations were 2.6 and 2.8 %, respectively.
AMH levels were measured and determined by first generation ELISA provided by DSL (Oxford Bio Innovation). The functional sensitivity of the assay was 0.05 ng/mL. Intra- and inter-assay coefficients of variation were <5 and <5.5 %, respectively. Conversion factor to ng/mL = pmol/L ÷ 7.143.
AMH was used in treatment individualisation and is presented in the three triage bands (15.6 pmol/L, 15.6–28.6 pmol/L, >28.6 pmol/L) used for clinical decision-making and allocation to a treatment protocol at that time.
On days 2–3 of a spontaneous menstrual cycle within 3 months of commencing ovarian stimulation, a transvaginal ultrasound scan was performed to assess the total number of antral follicles measuring 2–5 mm in diameter and to confirm normal anatomy of the pelvic organs. Intra-analysis coefficient of variation for follicular diameter measurements was <5 %, and the lower limit of detection was 0.5 mm.
IVF treatment protocol
Patients underwent either standard long downregulated cycles using gonadotrophin-releasing hormone (GnRH) analogues or short cycles using GnRH antagonists. The GnRH analogue (buserelin acetate; Aventis Pharma Ltd) was administered at the dose of 0.5 mg subcutaneously starting from the midluteal phase of the preceding menstrual cycle. Ovarian stimulation was effected with exogenous recombinant FSH (Puregon; Organon Laboratories Ltd) or highly purified FSH (Menopur; Ferring Pharmaceuticals). The GnRH analogue was reduced to 0.25 mg from the first day of gonadotrophin stimulation.
Patients on the antagonist cycle had gonadotrophin stimulation initiated on day 2 of the cycle, continuing up to and including the day of human chorionic gonadotrophin administration; the GnRH antagonist (Orgalutran; Organon Laboratories) at a daily dose of 0.25 mg was initiated using a fixed day-6 protocol.
Transvaginal ultrasounds were arranged on days 8 and 10, then daily or on alternate days thereafter as required. Final oocyte maturity was induced with 5000 IU of hCG (Pregnyl; Organon Laboratories) in the presence of more than three follicles of R17 mm. Oocyte retrieval was performed 34 to 36 hours after hCG administration, and a maximum of two embryos was transferred 3 days later. Vaginal progesterone pessaries (Cyclogest, 400 mg twice daily; Alpharma) were used to support the luteal phase. We adopted standard stimulation protocols across the groups.
Poor response was defined as the collection of less than four oocytes at retrieval or cancellation of the cycle when fewer than three mature follicles had developed. A normal response was considered to be retrieval of 4 to 20 oocytes, and over-response defined as >20 oocytes retrieved. Clinical pregnancy was defined as the presence of a gestation sac containing a foetal pole and foetal heart activity seen on transvaginal scan from 6 weeks of gestation.
Genotyping
DNA was extracted from blood samples using the Chemagen Automated DNA Separation System. Genotypes for AMH c.146G>T, p.(Ile49Ser) (rs10407022) and AMHR2 -482A>G (rs2002555) were determined using the TaqMan SNP pre-designed genotyping assay (assays ID C_25599842_10 and C_1673084_10; Applied Biosystems).
Statistical analysis
Statistical analysis was performed using Rv2.15 (R Development CoreTeam, 2013). Kruskall-Wallis and Fisher’s exact tests were used to compare characteristics across different genotypes.
Linear regression analysis was performed to assess the effect of the AMH c.146G>T and AMHR2 -482A>G genotypes, represented as an allele number, on the log of ovarian reserve markers (s-FSH, AFC and s-AMH), with adjustment for age (as a 4 degree of freedom cubic spline) and BMI (as a linear effect) and on the primary outcomes of response (gonadotrophin dose and log of number of oocytes retrieved) with adjustment for age, BMI and treatment (s-AMH treatment band, stimulation type and their interaction). s-AMH was used to select the treatment, hence its inclusion as a treatment parameter here. In addition, we also considered live birth and a dichotomous measure of over-response of >20 oocytes retrieved (used in a previous similar study [15]), which we analysed using analogous logistic regression models. There were no cycles cancelled for over-response, and any cycles cancelled for under-response were recorded as having yielded no oocytes.
A P value of <0.05 was considered statistically significant.
This study was performed using a previously collected and characterised sample; therefore, there was no formal sample size computation used to design the study. With genotypes containing the minor alleles forming approximately one-third of the sample, we have >80 % power to detect small effect sizes of 0.25 (in terms of the standard deviation between patients) or odds ratios of around 0.6 for a live birth rate of 30 %.
Results
Complete clinical data were available for 560 women: 19 were excluded as they never proceeded to ovarian stimulation, and no blood sample was obtained from 24 individuals. Genotyping of AMH c.146G>T, p.(Ile49Ser) and AMHR2 -482A>G variants was unsuccessful in 19 and 12 women, respectively.
Tables 1 and 2 show the characteristics/demographics for the women included in the analysis genotyped for AMH c.146G>T, p.(Ile49Ser) and AMHR2 -482A>G, respectively. There were no statistically significant differences in genotype frequencies with respect to BMI, duration of infertility or ovarian stimulation type. The one borderline significant association with AMHR2 and age (P = 0.041) would not be considered significant after allowing for the number of characteristics tested. Different allele frequencies in different ethnic groups were noted for AMH c.146G>T; therefore, we performed a separate analysis for the largest ethnic group (“White”), not presented here, which gave results consistent with the analysis for the whole group presented below.
There were no statistically significant associations between the measures of ovarian reserve (basal s-FSH, AFC and s-AMH) and the AMH c.146G>T or AMHR2 -482A>G genotype groups, after adjusting for age, ethnicity and BMI (Tables 3 and 4). There were also no significant associations between the genotypes and outcomes of ovarian response (in terms of number of oocytes and embryos retrieved, gonadotrophin dose and live birth rate) after adjustment for age, BMI and treatment.
A dichotomous measure of response (>20 oocytes for high response) was similarly analysed using equivalent logistic regression models. We did not find a statistically significant association between the AMH c.146G>T and AMHR2 -482A>G variants and high response to ovarian stimulation adjusted for age, ethnicity and BMI (per allele odds ratio (OR) = 0.77, 95 % confidence interval (CI) = 0.4–1.47 for AMH c.146G>T and OR = 0.83, CI = 0.41–1.65 for AMHR2 -482A>G, respectively).
Discussion
Controlled ovarian hyperstimulation in assisted reproduction involves the use of exogenous gonadotrophins, which can result in an excessive response including OHSS, or inadequate response leading to cycle cancellation. This significant variability in response has been the focus of many pharmacogenetic studies investigating single nucleotide polymorphisms (SNPs) as markers of ovarian reserve and predictors of ovarian response in order to optimise and individualise treatments.
Serum AMH levels are commonly used in current clinical practice as markers of ovarian reserve and to predict the response to controlled ovarian stimulation.
This large study investigated the association between the AMH c.146G>T, p.(Ile49Ser) and AMHR2 -482A>G polymorphisms and ovarian response in women undergoing IVF treatment. In addition, we included the most important clinical outcome, live birth rate, as an outcome measure. We did not identify any statistically significant differences (P < 0.05) in ovarian reserve markers and in treatment outcomes, including the number of oocytes retrieved and live birth rates between women with different AMH or AMHR2 genotypes, after adjusting our results for age, BMI, stimulation protocol and s-AMH band used to allocate treatment.
The study by Hanevik et al. similarly did not demonstrate any association between the AMH and AMHR2 polymorphisms and high or low response to ovarian stimulation [13]. Further, our study considered additional outcomes and markers of ovarian reserve.
Peluso et al. found in their study of 186 women that AMH polymorphisms were associated with the number of embryos produced and that AMHR2 polymorphisms were associated with the ovarian reserve markers (AMH, FSH levels and antral follicle count). However, they did not specify if any adjustments were made in their logistic regression model and so a direct comparison of the results is difficult.
Differences in results between association studies may be due to different allelic frequencies between ethnic groups. We did note differences in the allele frequencies between the ethnic groups recruited to our study and so performed a further analysis considering the dichotomous variables (over/under response) for the largest group (white British individuals) as per the previous study [13]. No significant associations were determined, indicating that it is unlikely that our combined analysis masked an association specific to a discrete ethnic group. Further, the allelic distributions we observed were similar to those found in Brazilian [Peluso et al.], Greek [Karagiorga et al.] and Norwegian [Hanevik et al.] cohorts.
Other factors that may explain the lack of consensus amongst the different studies that have investigated relationships between AMH and AMHR2 genotypes and controlled ovarian stimulation include differences in ovarian stimulation protocols used, the design of the study and the outcome measures. In the study by Karagiorga et al., significant associations were revealed only after subgroup analyses were performed based on age, AMH levels, peak E2 serum levels and number of IVF cycles. In addition, the outcome measure used in their study was different, as they compared number of follicles rather than number of oocytes retrieved used for our study.
Although the AMH and AMHR2 variants were not associated with ovarian response in this study, Boudjenah et al. found that it was only the combination of AMH and FSHR variants which correlated with the number of mature oocytes produced during controlled ovarian stimulation with recombinant FSH [17].
In conclusion, when considering the development of integrative clinical algorithms for individual FSH doses, our results indicate that genotyping AMH c.146G>T and AMHR2 -482A>G variants does not provide useful information as a predictor of response to ovarian stimulation and treatment outcomes.
References
Mohiyiddeen L, Nardo LG. Single-nucleotide polymorphisms in the FSH receptor gene and ovarian performance: future role in IVF. Hum Fertil (Camb). 2010;13(2):72–8.
Elgindy EA, El-Haieg DO, El-Sebaey A. Anti-Mullerian hormone: correlation of early follicular, ovulatory and midluteal levels with ovarian response and cycle outcome in intracytoplasmic sperm injection patients. Fertil Steril. 2008;89(6):1670–6.
La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum Reprod. 2006;21(12):3103–7.
Nelson SM, Yates RW, Fleming R. Serum anti-Müllerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles—implications for individualization of therapy. Hum Reprod. 2007;22(9):2414–21.
Rustamov O, Smith A, Roberts SA, Yates AP, Fitzgerald C, Krishnan M, et al. The measurement of anti-Müllerian hormone: a critical appraisal. J Clin Endocrinol Metab. 2014;99(3):723–32. doi:10.1210/jc.2013-3476.Epub2013Jan.
Visser JA, de Jong FH, Laven JS, Themmen AP. Anti-Mu¨llerian hormone: a new marker for ovarian function. Reproduction. 2006;131:1–9.
Kevenaar ME, Themmen AP, Rivadeneira F, Uitterlinden AG, Laven JS, van Schoor NM, et al. A polymorphism in the AMH type II receptor gene is associated with age at menopause in interaction with parity. Hum Reprod. 2007;22(9):2382–8.
Kevenaar ME, Themmen AP, Laven JS, Sonntag B, Fong SL, Uitterlinden AG, et al. Anti-Müllerian hormone and anti-Müllerian hormone type II receptor polymorphisms are associated with follicular phase estradiol levels in normo-ovulatory women. Hum Reprod. 2007;22(6):1547–54.
Voorhuis M, Broekmans FJ, Fauser BC, Onland-Moret NC, van der Schouw YT. Genes involved in initial follicle recruitment may be associated with age at menopause. J Clin Endocrinol Metab. 2011;96(3):E473–9.
Braem MG, Voorhuis M, van der Schouw YT, Peeters PH, Schouten LJ, Eijkemans MJ, et al. Interactions between genetic variants in AMH and AMHR2 may modify age at natural menopause. PLoS One. 2013;8(3):e59819.
Kevenaar ME, Laven JS, Fong SL, Uitterlinden AG, de Jong FH, Themmen AP, et al. A functional anti-mullerian hormone gene polymorphism is associated with follicle number and androgen levels in polycystic ovary syndrome patients. J Clin Endocrinol Metab. 2008;93(4):1310–6.
Rigon C, Andrisani A, Forzan M, D'Antona D, Bruson A, Cosmi E, et al. Association study of AMH and AMHRII polymorphisms with unexplained infertility. Fertil Steril. 2010;94(4):1244–8.
Hanevik HI, Hilmarsen HT, Skielbred CF, Tanbo T, Kahn JA. Single nucleotide polymorphisms in the anti-Müllerian hormone signalling pathway do not determine high or low response to ovarian stimulation. Reprod Biomed Online. 2010;21(5):616–23.
Karagiorga I, Partsinevelos GA, Mavrogianni D, Anagnostou E, Zervomanolakis I, Kallianidis K, et al. Single nucleotide polymorphisms in the Anti-Müllerian hormone (AMH Ile(49)Ser) and Anti-Müllerian hormone type II receptor (AMHRII −482 A > G) as genetic markers in assisted reproduction technology. J Assist Reprod Genet. 2015;32(3):357–67.
Peluso C, Fonseca FL, Gastaldo GG, Christofolini DM, Cordts EB, Barbosa CP, et al. AMH and AMHR2 polymorphisms and AMH serum level can predict assisted reproduction outcomes: a cross-sectional study. Cell Physiol Biochem. 2015;35(4):1401–12.
Cerra C, Oliver J, Roberts SA, Horne G, Newman WG, Mohiyiddeen L. A single nucleotide polymorphism of bone morphogenic protein-15 is not associated with ovarian reserve or response to ovarian stimulation. Hum Reprod. 2014;29(12):2832–7.
Boudjenah R, Molina-Gomes D, Torre A, Bergere M, Bailly M, Boitrelle F, et al. Genetic polymorphisms influence the ovarian response to rFSH stimulation in patients undergoing in vitro fertilization programs with ICSI. PLoS One. 2012;7(6):e38700.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule Genotyping of the AMH c.146G>T and AMHR2 -482A>G polymorphisms does not provide additional useful information as a predictor of ovarian reserve or ovarian response and treatment outcomes.
Stephen A. Roberts and Lamiya Mohiyiddeen contributed equally to this work.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Cerra, C., Newman, W.G., Tohlob, D. et al. AMH type II receptor and AMH gene polymorphisms are not associated with ovarian reserve, response, or outcomes in ovarian stimulation. J Assist Reprod Genet 33, 1085–1091 (2016). https://doi.org/10.1007/s10815-016-0711-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-016-0711-7