Abstract
Purpose
To quantify blastocyst morphologic parameters with a feasible and standardized tool, investigating their predictive value on implantation outcome.
Method
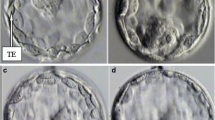
The study retrospectively analyzes 124 blastocysts from 75 patients. Quantitative measurements of blastocyst expansion, inner cell mass and trophoectoderm were taken using digital image analysis software.
Result(s)
Blastocysts areas were found to be ranging from 11626.2 up to 35076.4 μm2. The area of an early blastocyst is A ≤ 18500 μm2 with a mean diameter d = 140 ± 9 μm, and the area of an expanded blastocyst is A ≥ 24000 with d = 190 ± 9 μm. While blastocyst mean area was not related to implantation rate, more expanded blastocysts displayed a significantly higher implantation rate. Trophoectoderm cell number is a predictor of positive outcome: since a higher of cells (25.6 ± 11.3 vs 16.3 ± 12.8) `forming a tightly knit epithelium is prognostic of implantation potential. Conversely, inner cell mass size is significantly related to implantation only in expanded blastocysts (3122.7 ± 739.0 vs. 2978.1 ± 366.0 μm2).
Conclusion(s)
Evaluation of blastocyst morphology with a digital image system could be a valuable tool to standardize blastocyst grading based on quantitative parameters. Therefore, digital analysis may be helpful in identifying the best blastocyst to transfer.



Similar content being viewed by others
References
ESHRE. ESHRE ART fact sheet. http://www.eshre.eu/ESHRE/English/Guidelines-Legal/ART-factsheet/page.aspx/1061.
Fragouli J, Wells D. Aneuploidy in the human blastocyst. Cytogenet Genome Res. 2011;133:149–59.
Munne S. Chromosome Abnormalities and their relationship to morphology and development of human embryos. Reprod Biomed Online. 2006;12(2):234–54.
Braude P, Bolton V, Moore S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature. 1988;332:459–61.
Gardner DK, Schoolcraft WB. In vitro culture of human blastocyst. In: Janson R, Mortimer D, editors. Towards Reproductive Certainty: Infertility and Genetics Beyond 1999. Carnforth: Parthenon Press; 1999. p. 378–88.
Gardner DK, Schoolcraft WB, Wagley L, Schlenker T, Stevens J, Hesla J. A prospective randomized trial of blastocyst culture and transfer in in-vitro fertilization. Hum Reprod. 1998;13:3434–40.
Blake DA, Farquhar CM, Johnson N, Proctor M. Cleavage stage versus blastocyst stage embryo transfer in assisted conception. Cochrane Database Syst Rev. 2007;4:CD002118.
Papanikolaou EG, Kolibianakis EM, Tournaye H, Venetis CA, Fatemi H, Tarlatzis B, et al. Live birth rates after transfer of equal number of blastocysts or cleavage-stage embryos in IVF. A systematic review and meta-analysis. Hum Reprod. 2008;23:91–9.
Kirkegaard K, Hindkjaer J, Grøndahl M, Kesmodel U, Ingerslev H. A randomized clinical trial comparing embryo culture in a conventional incubator with a time-lapse incubator. J Assist Reprod Genet. 2012;29:565–72.
Cruz M, Gadea B, Garrido N, Pedersen KS, Martinez M, Perez-Cano I, et al. Embryo quality, blastocyst and ongoing pregnancy rates in oocyte donation patients whose embryos were monitored by time-lapse imaging. J Assist Reprod Genet. 2011;28:569–73.
Bendus AEB, Mayer JF, Shipley SK, Catherino WH. Interobserver and intraobserver variation in day 3 embryo grading. Fertil Steril. 2006;86:1608–15.
Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Reprod Biomed Online. 2011;22:632–46.
Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26(6):1270–83.
Hnida C, Engenheiro E, Ziebe S. Computer-controlled, multilevel, morphometric analysis of blastomere size as biomarker of fragmentation and multinuclearity in human embryos. Hum Reprod. 2004;19:288–93.
Ziebe S. Morphometric analysis of human embryos to predict developmental competence. Reprod Fertil Dev. 2013;26(1):55–64.
Paternot G, Debrock S, De Neubourg D, D’Hooghe TM, Spiessens C. Semi-automated morphometric analysis of human embryos can reveal correlations between total embryo volume and clinical pregnancy. Hum Reprod. 2013;28(3):627–33.
Beuchat A, Thevenaz P, Unser M, Ebner T, Senn A, Urner F, et al. Quantitative morphometrical characterization of human pronuclear zygotes. Hum Reprod. 2008;23:1983–92.
Santos Filho E, Noble JA, Wells D. A review on automatic analysis of human embryo microscope images. Open Biomed Eng J. 2010;4:170–7.
Santos Filho ES, Noble JA, Poli M, Griffiths T, Emerson G, Wells D. A method for semi-automatic grading of human blastocyst microscope images. Hum Reprod. 2012;27:2641–8.
De Kock AD, Smuts MP, Madden JD, Rodriguez AJ, Chantilis SJ, Meintjes M. Digital image analysis of blastocysts. Morphometrics correlations with pregnancy outcome. Fertil Steril. 2006;86:S51–2.
Ahlstrom A, Westin C, Reismer E, Wikland M, Hardarson T. Trophectoderm morphology: an important parameter for predicting live birth after single blastocyst transfer. Hum Reprod. 2011;26:3289–96.
Balaban B, Urman B, Sertac A, Alatas C, Aksoy S, Mercan R. Blastocyst quality affects the success of blastocyst-stage embryo transfer. Fertil Steril. 2000;74:282–7.
Dokras A, Sargent IL, Barlow DH. Human blastocyst grading: an indicator of developmental potential? Hum Reprod. 1993;8:2119–27.
Goto S, Kadowaki T, Tanaka S, Hashimoto H, Kokeguchi S, Shiotani M. Prediction of pregnancy rate by blastocyst morphological score and age, based on 1,488 single frozen-thawed blastocyst transfer cycles. Fertil Steril. 2011;95:948–52.
Hill MJ, Richter KS, Heitmann RJ, Graham JR, Tucker MJ, Decherney AH, et al. Trophectoderm grade predicts outcomes of single-blastocyst transfers. Fertil Steril. 2013;99(5):1283-9.
Honnma H, Baba T, Sasaki M, Hashiba Y, Ohno H, Fukunaga T, et al. Trophectoderm morphology significantly affects the rates of ongoing pregnancy and miscarriage in frozen-thawed single-blastocyst transfer cycle in vitro fertilization. Fertil Stertil. 2012;98:361–7.
Zaninovic N, Berrios R, Clarke RN, Bodine R, Ye Z, Veeck LL. Blastocyst expansion, inner cell mass (ICM) formation, and trophectoderm (TM) quality: is one more important for implantation? Fertil Steril. 2001;76:S8.
Richter KS, Harris DC, Daneshmand ST, Shapiro BS. Quantitative grading of a human blastocyst: optimal inner cell mass size and shape. Fertil Steril. 2001;76:1157–67.
Dal Prato L, Borini A, Trevisi MR, Bonu MA, Sereni E, Flamigni C. Effect of reduced dose of triptorelin at the start of ovarian stimulation on the outcome of IVF: a randomized study. Hum Reprod. 2001;16(7):1409–14.
Veeck LL, Zaninović N. An atlas of human blastocysts. New York: Parthenon Publishing Group; 2003.
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Thomas S. Large blastocyst diameter, early blastulation, and low preovulatory serum progesterone are dominant predictors of clinical pregnancy in fresh autologous cycles. Fertil Steril. 2008;90:302–9.
Campbell A, Fishel S, Bowman N, Duffy S, Sedler M, Hickman CF. Modelling a risk classification of aneuploidy in human embryos using non-invasive morphokinetics. Reprod Biomed Online. 2013;26(5):477–85.
Alfarawati S, Fragouli E, Colls P, Stevens J, Gutierrez-Mateo C, Schoolcraft WB, et al. The relationship between blastocyst morphology, chromosomal abnormality, and embryo gender. Fertil Steril. 2011;95:520–4.
Balaban B, Yakin K, Urman B. Randomized comparison of two different blastocyst grading systems. Fertil Steril. 2006;85:559–63.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule Blastocyst quantitative measurements are a useful tool to standardize morphological grading and highly indicative of embryo implantation potential.
Rights and permissions
About this article
Cite this article
Lagalla, C., Barberi, M., Orlando, G. et al. A quantitative approach to blastocyst quality evaluation: morphometric analysis and related IVF outcomes. J Assist Reprod Genet 32, 705–712 (2015). https://doi.org/10.1007/s10815-015-0469-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-015-0469-3