Abstract
Purpose
To investigate the predictive value of the motile sperm organelle morphology examination (MSOME) on embryo morphology.
Methods
The morphologies of 540 embryos obtained from 60 couples undergoing ICSI were evaluated from days 1 to 5 of development and were examined for associations with the percentages of morphologically normal paternal sperm and of the paternal sperm with large nuclear vacuoles (LNVs) as determined by MSOME.
Results
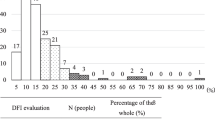
An increased percentage of LNV sperm was associated with increased odds of a zygote presenting with pronuclear abnormalities. It was also associated with decreased odds of (i) normal cleavage on days 2 and 3 of development, (ii) the presence of a high-quality embryo on day 3, (iii) the development of an embryo to the blastocyst stage, and (iv) an embryo possessing a normal trophectoderm and inner cell mass. The calculated areas under the curves differed for the embryos that did and did not develop to the blastocyst stage and for the high- and low-quality blastocysts. The optimal cut-off value for the percentage of LNV sperm that maximised proper blastocyst formation was ≤24.5 %, and the cut-off value that maximised blastocyst quality was ≤19.5 %.
Conclusions
These results suggest a very early onset of paternal influences on embryo development. The evaluation of the incidence of vacuoles by MSOME may significantly improve upon the prognostic information provided by conventional semen analyses.

Similar content being viewed by others
References
Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–8.
Shoukir Y, Chardonnens D, Campana A, Sakkas D. Blastocyst development from supernumerary embryos after intracytoplasmic sperm injection: a paternal influence? Hum Reprod. 1998;13:1632–7.
Loutradi KE, Tarlatzis BC, Goulis DG, et al. The effects of sperm quality on embryo development after intracytoplasmic sperm injection. J Assist Reprod Genet. 2006;23:69–74.
Virro MR, Larson-Cook KL, Evenson DP. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril. 2004;81:1289–95.
Janny L, Menezo YJ. Evidence for a strong paternal effect on human preimplantation embryo development and blastocyst formation. Mol Reprod Dev. 1994;38:36–42.
Miller JE, Smith TT. The effect of intracytoplasmic sperm injection and semen parameters on blastocyst development in vitro. Hum Reprod. 2001;16:918–24.
Van der Zwalmen P, Bertin-Segal G, Geerts L, Debauche C, Schoysman R. Sperm morphology and IVF pregnancy rate: comparison between Percoll gradient centrifugation and swim-up procedures. Hum Reprod. 1991;6:581–8.
Bartoov B, Berkovitz A, Eltes F. Selection of spermatozoa with normal nuclei to improve the pregnancy rate with intracytoplasmic sperm injection. N Engl J Med. 2001;345:1067–8.
Berkovitz A, Eltes F, Ellenbogen A, Peer S, Feldberg D, Bartoov B. Does the presence of nuclear vacuoles in human sperm selected for ICSI affect pregnancy outcome? Hum Reprod. 2006;21:1787–90.
Garolla A, Fortini D, Menegazzo M, et al. High-power microscopy for selecting spermatozoa for ICSI by physiological status. Reprod Biomed Online. 2008;17:610–6.
Boitrelle F, Ferfouri F, Petit JM, et al. Large human sperm vacuoles observed in motile spermatozoa under high magnification: nuclear thumbprints linked to failure of chromatin condensation. Hum Reprod. 2011;26:1650–8.
Setti AS, Cortezzi SS, Figueira Rde C, et al. A chromosome 19 locus positively influences the number of retrieved oocytes during stimulated cycles in Brazilian women. J Assist Reprod Genet. 2012;29:443–9.
World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: World Health Organization. xiv; 2010. 271.
Berkovitz A, Eltes F, Lederman H, et al. How to improve IVF-ICSI outcome by sperm selection. Reprod Biomed Online. 2006;12:634–8.
Saidi R, Rives N, Gruel E, Mazurier S, Mousset-Simeon N, Mace B. Nouvelle classification du spermocytogramme a’ fort grossissement. Med Reprod Gyn Endo. 2008;10:315–24.
Perdrix A, Travers A, Chelli MH, et al. Assessment of acrosome and nuclear abnormalities in human spermatozoa with large vacuoles. Hum Reprod. 2011;26:47–58.
Setti AS, de Almeida Ferreira Braga DP, Vingris L, de Cassia Savio Figueira R, Iaconelli Jr A, Borges Jr E. The prevalence of sperm with large nuclear vacuoles is a prognostic tool in the prediction of ICSI success. J Assist Reprod Genet. 2014;31:307–12.
Scott L, Alvero R, Leondires M, Miller B. The morphology of human pronuclear embryos is positively related to blastocyst development and implantation. Hum Reprod. 2000;15:2394–403.
Tesarik J, Greco E. The probability of abnormal preimplantation development can be predicted by a single static observation on pronuclear stage morphology. Hum Reprod. 1999;14:1318–23.
Alpha Scientists in Reproductive M, Embryology ESIGo. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–83.
De Vos A, Van De Velde H, Joris H, Verheyen G, Devroey P, Van Steirteghem A. Influence of individual sperm morphology on fertilization, embryo morphology, and pregnancy outcome of intracytoplasmic sperm injection. Fertil Steril. 2003;79:42–8.
Lundin K, Soderlund B, Hamberger L. The relationship between sperm morphology and rates of fertilization, pregnancy and spontaneous abortion in an in-vitro fertilization/intracytoplasmic sperm injection programme. Hum Reprod. 1997;12:2676–81.
Demir B, Arikan II, Bozdag G, Esinler I, Karakoc Sokmensuer L, Gunalp S. Effect of sperm morphology on clinical outcome parameters in ICSI cycles. Clin Exp Obstet Gynecol. 2012;39:144–6.
Braude P, Bolton V, Moore S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature. 1988;332:459–61.
Barroso G, Valdespin C, Vega E, et al. Developmental sperm contributions: fertilization and beyond. Fertil Steril. 2009;92:835–48.
Hinduja I, Baliga NB, Zaveri K. Correlation of human sperm centrosomal proteins with fertility. J Hum Reprod Sci. 2010;3:95–101.
Schatten H. The mammalian centrosome and its functional significance. Histochem Cell Biol. 2008;129:667–86.
Asch R, Simerly C, Ord T, Ord VA, Schatten G. The stages at which human fertilization arrests: microtubule and chromosome configurations in inseminated oocytes which failed to complete fertilization and development in humans. Hum Reprod. 1995;10:1897–906.
Terada Y, Nakamura S, Simerly C, et al. Centrosomal function assessment in human sperm using heterologous ICSI with rabbit eggs: a new male factor infertility assay. Mol Reprod Dev. 2004;67:360–5.
Tesarik J, Mendoza C, Greco E. Paternal effects acting during the first cell cycle of human preimplantation development after ICSI. Hum Reprod. 2002;17:184–9.
Tesarik J, Kopecny V. Nucleic acid synthesis and development of human male pronucleus. J Reprod Fertil. 1989;86:549–58.
Ao A, Erickson RP, Winston RM, Handyside AH. Transcription of paternal Y-linked genes in the human zygote as early as the pronucleate stage. Zygote. 1994;2:281–7.
Tesarik J, Sousa M, Testart J. Human oocyte activation after intracytoplasmic sperm injection. Hum Reprod. 1994;9:511–8.
Sathananthan A, The paternal centrosome: its role in human embryonic development and infertility, in Current issues in Obstetrics and Gynaecology, S. Arulkumaran and S. Ng, editors. Oxford University Press: Singapore, 1996. p. 101–16.
Ron-el R, Nachum H, Herman A, Golan A, Caspi E, Soffer Y. Delayed fertilization and poor embryonic development associated with impaired semen quality. Fertil Steril. 1991;55:338–44.
Parinaud J, Mieusset R, Vieitez G, Labal B, Richoilley G. Influence of sperm parameters on embryo quality. Fertil Steril. 1993;60:888–92.
Salumets A, Suikkari AM, Mols T, Soderstrom-Anttila V, Tuuri T. Influence of oocytes and spermatozoa on early embryonic development. Fertil Steril. 2002;78:1082–7.
Tesarik J. Paternal effects on cell division in the human preimplantation embryo. Reprod Biomed Online. 2005;10:370–5.
Tesarik J, Greco E, Mendoza C. Late, but not early, paternal effect on human embryo development is related to sperm DNA fragmentation. Hum Reprod. 2004;19:611–5.
Vingris L, Setti A, Braga D, Figueira R, Iaconelli Jr A, Borges Jr A. Motile sperm organelle morphology examination predicts blastocyst formation, implantation and miscarriage rates in couples undergoing ICSI. Hum Reprod. 2012;27:ii121–50.
Plastira K, Msaouel P, Angelopoulou R, et al. The effects of age on DNA fragmentation, chromatin packaging and conventional semen parameters in spermatozoa of oligoasthenoteratozoospermic patients. J Assist Reprod Genet. 2007;24:437–43.
de Almeida Ferreira Braga DP, Setti AS, Figueira RC, et al. Sperm organelle morphologic abnormalities: contributing factors and effects on intracytoplasmic sperm injection cycles outcomes. Urology. 2011;78:786–91.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule The proportion of sperm with large nuclear vacuoles negatively influences zygote, embryo and blastocyst development and quality.
Rights and permissions
About this article
Cite this article
Setti, A.S., Braga, D.P.A.F., Vingris, L. et al. Sperm morphological abnormalities visualised at high magnification predict embryonic development, from fertilisation to the blastocyst stage, in couples undergoing ICSI. J Assist Reprod Genet 31, 1533–1539 (2014). https://doi.org/10.1007/s10815-014-0326-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-014-0326-9