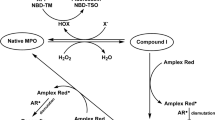
A study was carried out on the spectralluminescent properties of fl uorescein after its reaction with various reactive oxygen and halogen species (\({O}_{2}^{\bullet-},\) H2O2, HOCl, HOBr, HOSCN, N-chloramine, taurine N-chloramine, and taurine N-bromamine) as well as in the myeloperoxidase (MPO)–H2O2–Cl–/Br–/SCN– system. Reaction with only HOBr or with the MPO–H2O2–Br system turns fluorescein into a compound with an absorption maximum at 518 nm. The fluorescence maximum is recorded at 540 nm when excited at 520 nm, corresponding to eosin Y (brominated fluorescein). Conditions with phosphatebuffered saline (PBS) at pH 7.4 containing 137 mM NaCl, 5 mM fluorescein, 15–30 mM NaBr, and 25–50 mM H2O2 were found to be optimal for detecting HOBr in solution. A qualitative method for determining the brominating activity of MPO in vitro has been proposed. This method was used to study the effect of physiological and synthetic inhibitors as well as reactive oxygen and halogen species scavengers on the brominating activity of MPO. Our results indicate that fluorescein holds promise for use in a fluorescent method for detecting the brominating activity of mammalian hemecontaining peroxidases.
Similar content being viewed by others
References
J. Arnhold and E. Malle, Antioxidants (Basel), 11, No. 5, 890 (2022).
B. Bathish, R. Turner, M. Paumann-Page, A. J. Kettle, and C. C. Winterbourn, Arch. Biochem. Biophys., 646, 120–127 (2018).
H. B. Dunford, Redox Rep., 5, No. 4, 169–171 (2000).
P. G. Furtmüller, U. Burner, and C. Obinger, Biochemistry, 37, No. 51, 17923–17930 (1998).
D. I. Pattison and M. J. Davies, Curr. Med. Chem., 13, No. 27, 3271–3290 (2006).
O. M. Panasenko, I. V. Gorudko, and A. V. Sokolov, Usp. Biol. Khim., 53, 195–244 (2013).
Y. W. Yap, M. Whiteman, and N. S. Cheung, Cell Signal, 19, No. 2, 219–228 (2007).
M. Whiteman, J. P. Spencer, H. H. Szeto, and J. S. Armstrong, Antioxid. Redox Signal., 10, No. 3, 641–650 (2008).
O. M. Panasenko and V. I. Sergienko, Vest. Ros. Akad. Med. Nauk, No. 1, 27–39 (2010).
T. Nishikawa, E. Miyahara, M. Horiuchi, K. Izumo, Y. Okamoto, Y. Kawai, Y. Kawano, and T. Takeuchi, Environ. Health Perspect., 120, No. 1, 62–67 (2012).
R. Senthilmohan and A. J. Kettle, Arch. Biochem. Biophys., 445, No. 2, 235–244 (2006).
T. Suzuki, A. Nakamura, and M. Inukai, Bioorg. Med. Chem., 21, No. 13, 3674–3679 (2013).
O. M. Panasenko, T. Vakhrusheva, V. Tretyakov, H. Spalteholz, and J. Arnhold, Chem. Phys. Lipids, 149, 40–51 (2007).
M. J. Davies, J. Clin. Biochem. Nutr., 48, No. 1, 8–19 (2011).
M. J. Davies, C. L. Hawkins, D. I. Pattison, and M. D. Rees, Antioxid. Redox Signal., 10, No. 7, 1199–1234 (2008).
O. Skaff , D. I. Pattison, and M. J. Davies, Chem. Res. Toxic., 20, No. 12, 1980–1988 (2007).
V. E. Reut, I. V. Gorudko, D. V. Grigor'eva, A. V. Sokolov, and O. M. Panasenko, Bioorg. Khim., 48, No. 3, 1–27 (2022).
Y. Fang and W. Dehaen, Molecules, 26, No. 2, 363 (2021).
J. Flemmig, J. Zschaler, J. Remmler, and J. Arnhold, J. Biol. Chem., 287, No. 33, 27913–27923 (2012).
W. Qu, X. Zhang, Y. Ma, F. Yu, and H. Liu, Spectrochim. Acta A: Mol. Biomol. Spectrosc., 222, Article ID 117240 (2019).
X. Huo, X. Wang, R. Yang, Z. Li, Y. Sun, L. Qu, and H. Zeng, Sensors Actuators B: Chem., 315, Article ID 128125 (2020).
B. I. Stepanov, Introduction to the Chemistry and Technology of Organic Dyes [in Russian], Khimiya, Moscow (1984).
A. V. Sokolov, V. A. Kostevich, E. T. Zakharova, V. R. Samygina, O. M. Panasenko, and V. B. Vasilyev, Free Radic. Res., 49, 800–811 (2015).
A. V. Sokolov, V. A. Kostevich, D. N. Romanico, E. T. Zakharova, and V. B. Vasilyev, Biochemistry (Moscow), 77, No. 6, 631–638 (2012).
J. C. Morris, J. Phys. Chem., 70, 3798–3805 (1966).
K. Kumar and D. W. Margerum, Inorg. Chem., 26, No. 16, 2706–2711 (1987).
E. L. Thomas, P. M. Bozeman, M. M. Jeff erson, and C. C. King, J. Biol. Chem., 270, 2906–2913 (1995).
E. L. Thomas, M. B. Grisham, and M. M. Jeff erson, Methods Enzymol., 132, 569–585 (1986).
F. Y. Ge and L. G. Chen, J. Fluoresc., 18, 741–747 (2008).
A. V. Sokolov, V. A. Kostevich, S. O. Kozlov, I. S. Donskyi, I. I. Vlasova, A. O. Rudenko, E. T. Zakharova, V. B. Vasilyev, and O. M. Panasenko, Free Radic. Res., 49, No. 6, 777–789 (2015).
P. G. Furtmüller, U. Burner, W. Jantschko, G. Regelsberger, and C. Obinger, FEBS Lett., 484, 139–143 (2000).
M. PaumannPage, P. G. Furtmüller, S. Hofbauer, L. N. Paton, C. Obinger, and A. J. Kettle, Arch. Biochem. Biophys., 539, No. 1, 51–62 (2013).
A. L. Chapman, T. J. Mocatta, S. Shiva, A. Seidel, B. Chen, I. Khalilova, M. E. PaumannPage, G. N. Jameson, C. C. Winterbourn, and A. J. Kettle, J. Biol. Chem., 288, No. 9, 6465–6477 (2013).
A. V. Sokolov,, V. A. Kostevich, E. T. Zakharova, V. R. Samygina, O. M. Panasenko, and V. B. Vasilyev, Free Radic. Res., 49, No. 6, 800–811 (2015).
M. Roche, P. Rondeau, N. R. Singh, E. Tarnus, and E. Bourdon, FEBS Lett., 582, No. 13, 1783–1787 (2008).
A. J. Kettle, C. A. Gedye, and C. C. Winterbourn, Biochem. J., 321, 503–508 (1997).
B. Davies and S. W. Edwards, Biochem. J., 258, No. 3, 801–806 (1997).
P. R. Ortiz de Montellano, S. K. David, M. A. Ator, and D. Tew, Biochemistry, No. 15, 5470–5476 (1988).
P. M. Bozeman, D. B. Learn, and E. L. Thomas, Biochem. Pharmacol., 44, No. 3, 553–563 (1992).
D. I. Pattison and M. J. Davies, Curr. Med. Chem., 13, No. 27, 3271–3290 (2006).
J. D. Chandler and B. J. Day, Free Radic. Res., 49, No. 6, 695–710 (2015).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Prikladnoi Spektroskopii, Vol. 91, No. 2, pp. 234–244, March–April, 2024.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Grigorieva, D.V., Gorudko, I.V., Reut, V.E. et al. Detection of the Brominating Activity of Myeloperoxidase Using Fluorescein. J Appl Spectrosc 91, 313–322 (2024). https://doi.org/10.1007/s10812-024-01723-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-024-01723-x