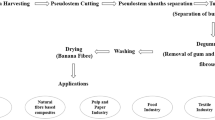
Current problems involving the use of flax product waste for obtaining products utilized for the production of cosmetics with sunscreen and antioxidant properties were examined. Ethanol lignin was obtained from flax shives as well as from raw materials subjected to alkaline pulping or biochemical modification. The bioprocessing included a stage of enzymatic degradation of the noncellulose polysaccharides to give aldoses and a stage of isothermal aging at 95°C. Chemical transformations in the polymer were studied by Fourier-transform infrared spectroscopy (FTIR) with resolution of the spectral curve into elementary vibrational bands of the main atomic groups. The spectra were analyzed using the C–C-stretching vibrational band of the aromatic ring at 1510 cm–1 as the internal standard. The enhanced absorption of the hydroxyaryl groups in the spectra of hydrolyzed lignin corresponds to the increase in intensity of the C=C-bond stretching and deformation vibrational bands. The increase in the auxochromic effect of the double bonds adjacent to the aromatic ring is seen in the increase in the absorption in the near-ultraviolet and visible light ranges. The biochemical preparation of the raw material led to disappearance of the IR bands for the double bonds in the carbonyl and alkene groups accompanied by a 1.5–2-fold enhancement of the absorption of alkyl hydroxyl groups and a 3–4-fold enhancement of the absorption of the hydroxyl group in the hydroxyaryl units. These results are consistent with the analytical data obtained upon biomodified preparation by the differential UV spectroscopic method and indicate greater photostabilizing capacity of lignin combined with absolute transparency in the visible range.
Similar content being viewed by others
References
M. V. Galkin and J. S. Samec, ChemSusChem., 9, No. 13, 1544–1558 (2016); https://doi.org/10.1002/cssc.201500237.
W. Schtyser, T. Renders, S. Van den Bosch, S. F. Koelewijn, G. T. Beckham, and B. F. Sels, Chem. Soc. Rev. 47, No. 3, 852–908 (2018); https://doi.org/10.1039/c7cs00566k.
T. I. Korányi, B. Fridrich, A. Pineda, and K. Barta, Molecules, 25, No. 12, 2815 (2020); https://doi.org/10.3390/molecules25122815.
L. Cao, I. K. M. Yu, Y. Liu, X. Ruan, D. C. W. Tsang, A. J. Hunt, Y. S. Ok, H. Song, and S. Zhang, Bioresources Technol., 269, 465–475 (2018); https://doi.org/10.1016/j.biotech.2018.08.065.
S. S. Wong, R. Shu, J. Zhang, H. Liu, and N. Yan, Chem. Soc. Rev., 49, No. 15, 5510–5560 (2020); https://doi.org/10.1039/d0cs00134a.
R. Zhang, C. H. Zhao, H. C. Chang, M. Z. Chai, B. Z. Li, and Y. J. Yuan, Eng. Life Sci., 19, No. 6, 463–470 (2019); https://doi.org/10.1002/elsc.201800133.
X. Liang, Q. Hu, X. Wang, L. Li, Y. Dong, C. Sun, C. Hu, and X. Gu, Polymers, 12, No. 9, 2123 (2020); https://doi.org/10.3390/polym12092123.
S. Nikafshar, J. Wang, K. Dunne, P. Sangthonganotai, and M. Nejad, ChemSusChem., 14, 1–13 (2021); https://doi.org/10.1002/cssc.202002729.
Q. Tang, Y. Qian, D. Yang, X. Qiu, Y. Qin, and M. Zhou, Polymers, 12, No. 11, 2471 (2020); https://doi.org/10.3390/polym12112471.
T. J. Szalaty, L. Klapiszewsk, and T. Jesionowski, J. Mol. Liq., 301, 112417 (2020); https://doi.org/10.1016/j.molliq.2019.112417.
H. Li, Y. Deng, J. Liang, Y. Dai, B. Li, Y. Ren, X. Qiu, and C. Li, Bioresources, 11, 3073–3083 (2016); https://doi.org/10.15376/biores.11.2.30733083.
M. P. Vinardell and M. Mityans, Int. J. Mol. Sci., 18, 1219 (2017); https://doi.org/10.3390/ijms18061219.
P. Morganti and M.B. Coltelli, Cosmetics, 6, No. 1 (2019); https://doi.org/10.3390/cosmetics6010010.
V. Martinez, M. Mitjans, and M. P. Binardelli, Currr. Organ. Chem., 16, 1863–1870 (2012), https://doi.org/10.2174/138527212802651223.
C. Corinaldesi, F. Marcellini, E. Nepote, E. Damiani, and R. Danovaro, Sci. Total. Environ., 637–638, 1279–1285 (2018); https://doi.org/10.1016/j.scitotenv.2018.05.108.
L. Ouchene, I. V. Litvinov, and E. Netchiporouk, J. Cutan. Med. Surg., 23, 648–649 (2019); https://doi.org/10.1177/1203475419871592.
A. Levine, Mar. Policy, 117, 103875 (2020); https://doi.org/10.1016/j.marpol.2020.103875.
O. Gordobil, P. Olaizola, J. M. Banales, and J. Labidi, Molecules, 25, No. 5, 1131 (2020); https://doi.org/10.3390/molecules25051131.
H. He, A. Li, S. Li, J. Tang, L. Li, and L. Xiong, Biomed. Pharmac., 134, 111161 (2021); https://doi.org/10.1016/j.biopha.2020.111161.
D. Piccinino, E. Capecchi, E. Tomaino, S. Gabellone, V. Gigli, D. Avitabile, and R. Saladino, Antioxidants, 10, No. 2, 274 (2021); https://doi.org/10.3390/antiox10020274.
M. E. Vallejos, F. E. Felissia, A. A. S. Curvelo, M. D. Zambon, L. Ramos, and M. C. Area, Bioresources, 1158–1171 (2011); https://doi.org/10.15376/biores.6.2.11581171.
S. Laurichesse and L. Averous, Prog. Polym. Sci., 39, No. 7, 1266–1290 (2014), https://doi.org/10.1016/j.progjoplymsci.2013.11.004.
Y. Qian, X. Q. Qiu, and S. P. Zhu, Green Chem., 17, No. 1, 320–324 (2015); https://doi.org/10.1039/C4FC01333F.
A. Duval and M. Lawoko, React. Funct. Polym., 85, 78–96 (2014); https://doi.org/10.1016/j.reactfunctpolym.2014.09.017.
S. R. Yearla and K. Padmasree, J. Exp. Nanosci., 10, No. 18, 1–14 (2015); https://doi.org/10.1080/17458080.2015.1055842.
D. Tian, J. Hu, J. Bao, B. P. Chandra, J. N. Saddler, and C. Lu, Biotechnol. Biofuels, 10, 192 (2017); https://doi.org/10.1186/s130680170876z.
J. Wang, Y. Deng, Y. Qian, X. Qiu, Y. Ren, and D. Yang, Green Chem., 18, 695–699 (2016); https://doi.org/10.1039/C5GC02180D.
H. Zhang, X. Liu, S. Fu, and Y. Chen, Int. J. Biol. Macromol., 133, 86–92 (2019); https://doi.org/10.1016/j.ijbiomac.2019.04.092.
S. C. Lee, T. M. T. Tran, J. W. Choi, and K. Won, Int. J. Biol. Macromol., 122, 549–554 (2019); https://doi.org/10.1016/j.ijbiomac.2018.10.184.
O. Lepilova, G. Spigno, S. Aleeva, and S. Koksharov, Eurasian Chem. Technol. J., 19, No. 1, 31–40 (2017); https://doi.org/10.18321/ectj500.
O. V. Lepilova, S. V. Aleeva, and S. A. Koksharov, Russ. J. Org. Chem., 48, No. 1, 83–88 (2012); https://doi.org/10.1134/s1070428012010125.
S. Koksharov, S. Aleeva, and O. Lepilova, Autex Res., 15, No. 3, 215–225 (2015); https://doi.org/10.1515/aut20150003.
W. J. J. Huijgen, A. T. Smit, P. J. de Wild, and H. den Uil, Bioresource Technol., 114, 389–398 (2012); https://doi.org/10.1016/j.biortech.2012.02.143.
S. V. Aleeva and S. A. Koksharov, Russ. J. Gen. Chem., 82, No. 13, 2279–2293 (2012); https://doi.org/10.1134//S1070363212130154.
E. Pretsch, P. Buhlmann, and M. Badertscher, Structure Determination of Organic Compounds, Springer-Verlag, Berlin–Heidelberg (2009).
N. G. Bazarnovaya (Ed.), Research Methods for Wood and Its Derivatives [in Russian], Barnaul, Altai State University (2002).
O. Yu. Derkacheva, J. Appl. Spectrosc., 80, No. 5, 670–676 (2013); https://doi.org/10.1007/s108120139825.
S. V. Aleeva, O. V. Lepilova, and S. A. Koksharov, J. Appl. Spectrosc., 87, No. 5, 779–783 (2020); https://doi.org/10.31857/d0044453720060035.
M. Khazma, A. Goullieux, R. M. Dheilly, A. Rougier, and M. Quenuedec, Ind. Crop. Prod., 61, 442–452 (2014); https://doi.org/10.1016/j.indcrop.2014.07.041.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Prikladnoi Spektroskopii, Vol. 88, No. 4, 603–610, July–August, 2021.
Rights and permissions
About this article
Cite this article
Aleeva, S.V., Lepilova, O.V. & Koksharov, S.A. Chemical Transformations of Flax Shive Lignin by the Action of Polysaccharide Fermentation Products. J Appl Spectrosc 88, 781–788 (2021). https://doi.org/10.1007/s10812-021-01240-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-021-01240-1