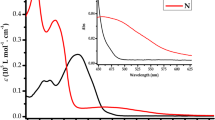
The complexation of recently synthesized symmetrical bifunctional bis-azospiropyran photochromic dye with europium nitrate and its effect on UV–vis absorption and fluorescent emission was studied. Upon addition of Eu 3+ to colorless spiropyran, a yellow merocyanine europium complex was obtained with an absorption band at 410 nm. Negatively charged phenolic oxygenin zwitterionic ring-open form provides an effective metal binding site for Eu 3+ . Meanwhile, the inherent fluorescence emission of the photochromic dye at 380 nm is switched off due to the Eu 3+ - induced drive of spiro-mero equilibrium to form mero form. The stoichiometry of dye–europium complexation was evaluated by fluorescence emission and UV–vis absorption spectroscopy and a 8:1 ratio was obtained in both cases. The binding constant (K) value of the dye–europium complex was 3 × 106 M −1 . In conclusion, the current molecular switch is a useful sensitive dual measuring tool for solutions containing europium or europium-like elements by evaluation of visible absorption or fluorescent emission spectroscopy.
Similar content being viewed by others
References
P. Norouzi, M. Hosseini, M. R. Ganjali, M. Rezapour, and M. Adibi, Int. J. Electrochem. Sci., 6, 2012–2021 (2011).
Y. Liu, H. Dong, W. Zhang, Z. Ye, G. Wang, and J. Yuan, Biosens. Bioelectron., 25, 2375–2378 (2010).
Ch. Tanb, Q. Wanga, C. Tan, and Q. Wang, Synth. Methods, 162,1416–1420 (2012).
G. Shaoa, Y. Li, K. Feng, F. Gan, and M. Gonga, Sens. Actuators, B, 173, 692–697 (2012).
J. Li, S. Liu, X. Mao, P. Gao, and Z. Yan, J. Electroanal. Chem., 561, 137–142 (2004).
H. Iwanaga, A. Amano, F. Aiga, K. Harada, and M. Oguchi, J. Alloys Compd., 408–412, 921–925 (2006).
F. Aiga, H. Iwanaga, and A. Amano, J. Phys. Chem. A, 109, 11312–11316 (2005).
P. He, H. H. Wang, S. G. Liu, W. Hu, J. X. Shi, G. Wang, and M. L. Gong, J. Electrochem. Soc., 156, 46–49 (2009).
K. Järås, A. A. Tajudin, A. Ressine, T. Soukka, G. Marko-Varga, A. Bjartell, J. Malm, T. Laurell, and H. Lilja, J. Proteome Res., 7, 1308–1314 (2008).
C. Huang, Z. Jiang, and B. Hu, Talanta, 73, 274–281 (2007).
C. Karadas, D. Kara, and A. Fisher, Anal. Chim. Acta, 689, 184–189 (2011).
K. Mostafavi, M. Ghahari, S. Baghshahi, and A. M. Arabi, J. Alloys Compd., 555, 62–67 (2013).
N. Arnaud and J. Georges, Analyst, 122, 143–146 (1997).
Sh. Yagi, Sh. Nakamura, D. Watanabe, and H. Nakazumi, Dyes Pigm., 80, 98–105 (2009).
S. G. Kandi and F. Nourmohammadian, J. Mol. Struct., 1050, 222–231 (2013).
R. Guglielmetti, In "Photochromism:Molecules and Systems", Ed. H. Durr, H. Bouas-Laurent, ch. 8, 23, Amsterdam, Elsevier (1990).
E. Mukhanov, Y. Alekseenko, B. Luk’yanov, I. Dorogan, and S. Bezugly, High Energ. Chem., 44, 220–223 (2010).
J. Buback, M. Kullmann, F. Langhojer, P. Nuernberger, R. Schmidt, F. Wurthner, and T. Brixner, J. Am. Chem. Soc., 132, 16510–16519 (2010).
F. Nourmohammadian and A. Ashtiani Abdi, Bull. Korean Chem. Soc., 34, 1727–1734 (2013).
M. Ghahari, P. Fabbri, F. Pilati, L. Pasquali, M. Montecchi, and R. Aghababazadeh, Ceram. Int., 39, 4513–4521 (2013).
M. Natali, L. Soldi, and S. Giordani, Tetrahedron, 66, 7612–7617 (2010).
N. Shao, Y. Zhang, S. M. Cheung, R. H. Yang, W. Chan, T. Mo, K. A. Li, and F. Liu, Anal. Chem., 77, 7294–7303 (2005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Zhurnal Prikladnoi Spektroskopii, Vol. 82, No. 4, pp. 526–531, July–August, 2015.
Rights and permissions
About this article
Cite this article
Nourmohammadian, F., Ghahari, M. & Gholami, M.D. Spectral Properties of a bis-Azospiropyran Complexed with Europium. J Appl Spectrosc 82, 561–566 (2015). https://doi.org/10.1007/s10812-015-0145-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-015-0145-5