Abstract
Seaweed aquaculture, which takes place mostly in Asia, is a lucrative industry that is valued > US $9 billion. However, technological modifications are needed to ensure economic viability and growth of the seaweed aquaculture industry throughout Europe. While current research is investigating the use of certain mechanised processes in seaweed aquaculture, the impact of pressurised spraying of macroalgal cultures on subsequent growth remains unknown. Here, we aimed to determine the efficacy of a future mechanised seeding procedure by investigating how differing pressure treatments impact upon the growth and percentage cover of zoospores seeded onto twine in the hatchery, using the kelp Saccharina latissima as a model species. Zoospore solutions were subjected to pressures of 1, 2, 3, 4, and 5 bar, before being seeded on hatchery twine and left to grow for 7 weeks. We demonstrate that both percentage cover and sporophyte lengths for S. latissima are significantly reduced by ~ 22% and ~ 61%, respectively, when juvenile zoospores are subjected to increasing pressure from 1 to 5 bar. This indicates that minimal pressure in the use of mechanised hatchery techniques is optimal for growth of seaweed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In terms of food production, marine aquaculture is now the fastest growing sector globally, making it one of the most promising industries for future food security (Duarte et al. 2022). More specifically, seaweed aquaculture, which takes place mostly in Asian countries such as China and Indonesia, is a lucrative industry with a projected growth of up to US$11.8 billion by 2030 (World Bank 2023). Harvesting seaweed from aquaculture not only provides a valuable food source for a growing world population, but also supplies a range of industries and their development of essential materials such as cosmetics, fertilisers, food additives, and pharmaceuticals (Hasselström et al. 2018). In addition, seaweed aquaculture could have a range of environmental benefits, such as providing protection against coastal erosion by reducing wave energy (Løvås and Tørum 2001). Also, by acting as a ‘biological filter’ for the global oceans, seaweed can absorb large quantities of nutrients during growth and produce oxygen, which could help to improve coastal waters where eutrophication is an issue (Hu et al. 2022). Importantly, when produced on an industrial scale, seaweeds may help to mitigate against the impacts of global climate change, as they sequester large quantities of CO2 (Duarte et al. 2013; Zheng et al. 2019).
Despite the environmental and economic benefits of the expanding seaweed aquaculture industry, it remains only a marginally economically viable activity throughout Europe (Zheng et al. 2019). Several European countries including Ireland, Norway, and Sweden are supporting research that focuses on seaweed cultivation techniques to aid accessibility for the European market (Handå et al. 2013; Forbord et al. 2020a, b; Kerrison et al. 2020). Seaweed is generally grown using long-line techniques which can be highly labour intensive (Peteiro et al. 2016; Campbell et al. 2019). Therefore, technological modifications are needed to ensure economic viability for the seaweed aquaculture industry to grow throughout Europe (Edwards and Watson 2011). These modifications are not only needed to reduce labour costs in the harvesting of seaweed biomass, but also to reduce the hatchery phase where zoospore or gametophyte cultures are seeded onto twine and grown in tanks for a minimum of four weeks prior to their introduction to coastal seas (see Edwards and Watson 2011; Campbell et al. 2019; Kerrison et al. 2020).
Consequently, the scope to expand seaweed aquaculture throughout Europe, and indeed globally, will be limited by the high production costs associated with labour and the processes involved during the hatchery growth phases (Forbord et al. 2020a, b). For reference, a detailed description of the different hatchery processes is outlined in the best practise report for seaweed cultivation by Mooney-McAuley et al. (2016). However, mechanising parts of the aquaculture industry is being seen as a way of increasing the viability of the seaweed market (Werner et al. 2004). While current research is investigating the efficacy of mechanised processes including the deployment of seedlings from twine to ropes, biomass harvesting and crop handling logistics (Stévant et al. 2017), the impact of mechanised and pressurised spraying of seaweed cultures on growth are yet to be established. Mechanised spraying of substrata with gametophyte cultures during the initial hatchery growth phase (Mooney-McAuley et al. 2016) could reduce the time and labour costs of preparing large quantities of spools for cultivation purposes, however an initial investigation into the effects of pressure on kelp zoospores is needed. Hence, in this study we aimed to determine the efficacy of a future mechanised seeding procedure by investigating how differing pressure treatments impact upon the growth of Saccharina latissima zoospores when they are seeded onto twine in the hatchery.
Methods
Sample collection and spore release
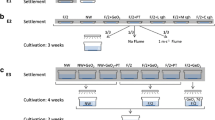
Ten fertile drift sporophytes of Saccharina latissima were collected from Kilclief beach, Northern Ireland (54.3359° N, 5.5435° W) on 24 October 2021. The S. latissima sporophytes were wiped clean and placed on paper wetted with seawater before being enclosed in plastic bags and refrigerated overnight (4 °C).
Following a 24-h period, sporangial areas were excised from each individual blade and randomly divided between five containers to a total mass of 75 g per container. At 10 min intervals, to allow for time to carry out experimental treatments (see below), each batch of 75 g was added to a 1250-mL beaker containing autoclaved seawater and placed on an orbital shaker (Stuart SSL1, Davidson & Hardy Ltd, UK) at 150 rpm to release spores.
Experiment
After 30 min of spore release on the orbital shaker, 200 mL of the resulting spore solution was filtered through 45-µm nylon mesh and funnelled into a clean 1-L pressure cylinder (North Sea Winches Ltd, Scarborough, UK). The solution was then subjected to a pressure treatment for 5 min before being poured into a 250-mL conical flask. This procedure was then repeated six times for each of five pressure treatments (N = 30): 1 (control), 2, 3, 4 and 5 bar (or 100, 200, 300, 400, and 500 kPa). The pressure cylinder, funnel and filter were rinsed with deionised water between each replicate.
Simultaneously, spores were visually checked for motility under a light microscope (Leica DMIRB) and 9 mL of each of the five spore solutions was added to a falcon tube with 1 mL of 7% Lugol’s iodine (Sigma-Aldrich; Product no. L6146) for analysis of spore density mL–1 in the resulting 0.1% (v/v) solution. Subsequent spore counts using a compound microscope (Olympus BX41) and haemocytometer (Hawksley, England, UK) (Guillard and Sieracki 2005) revealed mean (± SE) spore densities of 31,666 ± 5068 mL–1 (1 bar), 54,333 ± 11,181 mL–1 (2 bar), 33,333 ± 10,833 mL–1 (3 bar), 56,000 ± 10,981 mL–1 (4 bar) and 11,666 ± 3333 mL–1 (5 bar). While the exceptionally low spore density in the solution used for the 5 bar treatment requires careful attention in the interpretation of our results for that treatment, the other densities are fairly consistent and randomly distributed among treatments.
Each flask was equipped with cotton twine (10 cm lengths) and placed under a white 12:12 light cycle (20 µmol photons m–2 s–1) in a temperature-controlled room (12 °C). Spores were left to settle on the twine for 2 days with no airflow. Following this, the flasks were emptied, and the twine was suspended in new flasks containing 200 mL of continuously aerated autoclaved seawater, supplemented with 0.1 mL germanium dioxide (104.61 g mol−1; Sigma-Aldrich; CAS no. 1310–53-8) to reduce diatom growth, and 0.2 mL of 10% F/2 medium (Sigma Aldrich; Product no. G0154) for nutrients, and left to grow for 7 weeks. Water changes and replenishment of nutrients was carried out twice per week.
Quantification
Following completion of the experiment after 7 weeks, three 1-cm sections (middle and ends) of each 10-cm twine (N = 30) were photographed using an Olympus SZX16 stereomicroscope with an Olympus E600 DSLR camera attachment. All photographs were taken at 10 × magnification with a 10-mm object for scale. Using Coral Point Count v4.1 with Excel extensions software (Kohler and Gill 2006), a 10 mm quadrat was added to each photograph and the percentage cover of S. latissima was calculated by assigning 10 random points (< 1 mm in size) within the quadrat and recording presence or absence. These steps resulted in a binomial response variable for sporophyte cover with 30 × 3 × 10 = 900 technical replicates (ones and zeros).
To corroborate estimated sporophyte cover, up to five of the longest sporophytes from each replicate were measured using ImageJ v1.53 k (Rasband 2012). Nine replicates did not have measurable sporophytes leading to a reduced sample size (N = 21). These steps resulted in a continuous response variable for sporophyte length with 101 technical replicates.
Data analysis and visualisation
Data were analysed and visualised in R v4.2.1 (R Core Team 2022) within the integrated development environment RStudio v2022.12.0 + 353 (RStudio Team 2022). We modelled the effects of pressure on sporophyte cover and length using generalised linear mixed models (GLMMs) written with the glmer function of lme4 v1.1–31 (Bates et al. 2015). Multilevel modelling was strictly necessary to counteract artificial inflation of sample size and concurrently confidence through our extensive technical replication. Using the hierarchical structure of GLMMs, we nested technical replicates within independent statistical ones by defining flask as a random factor, allowing the model intercept to vary among flasks and adding this additional source of uncertainty to our estimation of confidence. We did not choose to factor variation in spore density of the inoculating solutions into our models since this would have convoluted them and no potential confounding effect was evident (see Sect. "Experiment").
The binomial (presence, absence) nature of our cover variable dictated a binomial GLMM for which we selected the logistic link function. Since binomial data are not symmetrically distributed around the mean and normal approximation (Wald) is consequently suboptimal, asymmetric Wilson score 95% confidence intervals and s.e.m. were calculated with binom v1.1–1 (Dorai-Raj 2015). Sporophyte length was right-skewed like most biological response variables but only takes positive values so was perfectly suited for a gamma GLMM with a logarithmic link function. Model optimality was determined based on graphical scrutiny of residuals for normality and homogeneity (Zuur et al. 2009).
Type II Wald chi-square (χ2) tests of the effect of pressure – testing the null hypothesis that the slope is not different from zero – were performed with the Anova function of car v3.1–1 (Fox & Weisberg 2019). Data were visualised in ggplot2 v3.3.3 (Wickham 2016), plots aligned in cowplot v1.1.1 (Wilke 2020) and illustrations added in Affinity Designer v1.10.6 (Serif Ltd, Nottingham, UK). All analysis and visualisation steps can be scrutinised and replicated using the files provided at github.com/lukaseamus/spores-under-pressure.
Results
Sporophyte growth was visually confirmed in all treatments over the course of the 7-week experiment (Fig. 1). Our model suggests that the percentage cover (c) of S. latissima (%) decreases with increasing pressure (p) (N = 30, χ21,30 = 13.36, p < 0.001, Fig. 2a), a trend which is best described by \({\textit{c}}= \frac{100}{{1}\mathrm{+}{\mathrm{e}}^{\mathrm{0.33}{\mathrm{p}}\mathrm{ + }\mathrm{0.12}}}\). Across our range of pressure treatments, c decreased by 22.22% from 41.67 [24.19, 61.53] % (mean [– s.e.m., + s.e.m.], n = 6) at 1 bar (ambient atmospheric pressure) to 19.44 [8.23, 39.39] % at 5 bar, although it reached its minimum with 17.78 [7.21, 37.55] % at 4 bar. The largest stepwise decrease between pressure treatments (10%) was observed from ambient to 31.67 [16.51, 52.06] % at a doubling of atmospheric pressure (2 bar).
The percentage cover (a) and lengths (b) of Saccharina latissima sporophytes under increasing pressure. N = total technical (statistical) sample size. Points are technical replicates (measurements) and point-ranges are statistical replicates (flasks) and their corresponding measurement error (technical 95% confidence intervals). Lines are model fits and shaded areas are 95% confidence intervals, augmented with intercept variance between flasks as an additional source of uncertainty
Length (l) of individual S. latissima sporophytes (mm) also decreases with increasing pressure according to our model (N = 21, χ21,21 = 7.60, p = 0.006, Fig. 2b), a trend which is best described by \({\mathrm{l}} \, \mathrm{=}{ \, {\mathrm{e}}}^{-\mathrm{0.23}{\mathrm{p}}\mathrm{+}\mathrm{0.12}}\). These findings corroborate the pressure effect on c. Across our range of pressure treatments, l decreased by 0.53 mm from 0.87 ± 0.11 mm (mean ± s.e.m., n = 6) at ambient pressure to its minimum 0.34 ± 0.04 mm (n = 5) at 5 bar, although it reached its maximum with 0.93 ± 0.4 mm (n = 3) at 3 bar. The largest stepwise decrease between pressure treatments (0.52 mm) was observed from 3 bar to 0.41 ± 0.11 (n = 2) at 4 bar.
Discussion
Here we demonstrate that both S. latissima percentage cover and sporophyte lengths are significantly reduced when zoospores are subjected to pressure at the start of the hatchery growing stage. Our results highlight that S. latissima grew more effectively and in higher numbers over 7 weeks when zoospores were only exposed to normal atmospheric pressure, and therefore our models indicate that minimal pressure is ideal for future mechanised hatchery techniques.
For this study we adopted a direct seeding approach, whereby viable zoospores are directly attached to the substrata upon release from the fertile blade material (Mooney-McAuley et al. 2016) to determine the percentage growth coverage at increasing pressures. This form of direct seeding (not to be confused with binder-seeding techniques; Forbord et al. 2020b) has been highlighted as the most cost effective, favourable and simplest seeding approach used in the aquaculture of S. latissima (Mooney-McAuley et al. 2016). Also, while natural fertile material can only be used at certain times of the year (Mooney-McAuley et al. 2016), year-round sorus induction of S. latissima is possible by manipulating light levels (Forbord et al. 2012). Despite only obtaining 40% sporophyte coverage in our study at 1 bar, compared to the ~ 80% identified in Forbord et al. (2020b) over the same period, different methods were used to calculate percentage cover which may explain the discrepancy. Further, zoospore densities of ~ 250,000 mL−1 were reported in Forbord et al. (2020b), whereas in our study, much lower densities (~ 11,000—~ 56,000 mL−1) were observed in our pressure treatment samples. Although zoospore densities fluctuated from high to low across our five pressure treatments, a decreasing correlation of percentage sporophyte cover and lengths was still found at the end of the experiment.
While we have determined the effects of increasing pressure on S. latissima zoospores in this study, investigation into the impacts on the gametophyte stage would be beneficial. This would help in determining the broader effects of pressure, especially in terms of spraying cultures onto cultivation materials. This is particularly important as the gametophyte stage of the kelp lifecycle can be used to seed lines all year round by storing them under red light where they continue to increase in density through cellular division (Lüning 1979). It also would be beneficial to monitor the zoospore germination and onward development of the kelp after pressure treatments to determine if the negative effects found here occur immediately or as development of the spores continues over time. To further understand the cost benefit analysis of using a mechanised gametophyte spraying approach in comparison to manual spraying, a follow up assessment of growth in the field and an investigation into optimal seeding densities for successful growth is also needed. Particularly, as studies suggest that lab-based results do not necessarily translate to the field (Kerrison et al. 2019) and that the optimal density of gametophytes or sporophytes on substrata for any seaweed species remains unknown (Kerrison et al. 2015). This would help to determine if the pressure treatments impede growth after the hatchery stage and could be tested using both direct seeding of zoospores and seeding of gametophytes. Finally, an investigation into the effect of mechanical and physical stress of mechanised spraying on zoospores and gametophytes may also be beneficial.
In conclusion, our models highlight that sporophyte cover and length declines with increasing pressure applied during seeding, indicating that minimal pressure is optimal for sporophyte growth in future mechanised hatchery techniques. Further research is also needed to determine how increasing pressure impacts upon the growth of the mature gametophyte life stage in a hatchery setting. A follow-up validation study is also needed to determine how hatchery pressure treatments impact upon the subsequent growth of S. latissima sporophytes in the field.
Data availability
Data alongside the annotated R script are available from the open-access repository at github.com/lukaseamus/spores-under-pressure.
Code availability
Data alongside the annotated R script are available from the open-access repository at github.com/lukaseamus/spores-under-pressure .
References
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67: 1–48
Campbell I, Macleod A, Sahlmann C, Neves L, Funderud J, Øverland M, Hughes AD, Stanley M (2019) The environmental risks associated with the development of seaweed farming in Europe-prioritizing key knowledge gaps. Front Mar Sci 6:107
Dorai-Raj S (2015) binom: binomial confidence intervals for several parameterizations. R package v1.1–1 https://CRAN.R-project.org/package=binom
Duarte CM, Bruhn A, Krause-Jensen D (2022) A seaweed aquaculture imperative to meet global sustainability targets. Nat Sustain 5:185–193
Duarte CM, Losada IJ, Hendriks IE, Mazarrasa I, Marbà N (2013) The role of coastal plant communities for climate change mitigation and adaptation. Nat Clim Change 3:961–968
Edwards M, Watson L (2011) Aquaculture Explained. No. 26 Cultivating Laminaria digitata. Irish Sea Fisheries Board, Dublin
Forbord S, Skjermo J, Arff J, Handå A, Reitan KI, Bjerregaard R, Lüning K (2012) Development of Saccharina latissima (Phaeophyceae) kelp hatcheries with year-round production of zoospores and juvenile sporophytes on culture ropes for kelp aquaculture. J Appl Phycol 24:393–399
Forbord S, Matsson S, Brodahl GE, Bluhm BA, Broch OJ, Handå A, Metaxas A, Skjermo J, Steinhovden KB, Olsen Y (2020a) Latitudinal, seasonal and depth-dependent variation in growth, chemical composition and biofouling of cultivated Saccharina latissima (Phaeophyceae) along the Norwegian coast. J Appl Phycol 32:2215–2232
Forbord S, Steinhovden KB, Solvang T, Handå A, Skjermo J (2020b) Effect of seeding methods and hatchery periods on sea cultivation of Saccharina latissima (Phaeophyceae): a Norwegian case study. J Appl Phycol 32:2201–2212
Fox J, Weisberg S (2019) An R companion to applied regression. Sage, Thousand Oaks. https://socialsciences.mcmaster.ca/jfox/Books/Companion/. Accessed Dec 2022
Guillard RRL, Sieracki MS (2005) Counting cells in cultures with the light microscope. In: Anderson RA (ed) Algal Culturing Techniques. Elsevier Academic Press, London, pp 239–252
Handå A, Forbord S, Wang X, Broch OJ, Dahle SW, Størseth TR, Reitan KI, Olsen Y, Skjermo J (2013) Seasonal-and depth-dependent growth of cultivated kelp (Saccharina latissima) in close proximity to salmon (Salmo salar) aquaculture in Norway. Aquaculture 414:191–201
Hasselström L, Visch W, Gröndahl F, Nylund GM, Pavia H (2018) The impact of seaweed cultivation on ecosystem services-a case study from the west coast of Sweden. Mar Pollut Bull 133:53–64
Hu S, Zou D, He Q, Shi X, Liu L (2022) Evaluation for values of ecosystem service functions of cultivated seaweeds in Guangdong Province. China Algal Res 63:102657
Kerrison PD, Stanley MS, Edwards MD, Black KD, Hughes AD (2015) The cultivation of European kelp for bioenergy: site and species selection. Biomass Bioenergy 80:229–242
Kerrison PD, Innes M, Macleod A, McCormick E, Elbourne PD, Stanley MS, Hughes AD, Kelly MS (2020) Comparing the effectiveness of twine-and binder-seeding in the Laminariales species Alaria esculenta and Saccharina latissima. J Appl Phycol 32:2173–2181
Kerrison PD, Twigg G, Stanley M, De Smet D, Buyle G, Martínez Pina A, Hughes AD (2019) Twine selection is essential for successful hatchery cultivation of Saccharina latissima, seeded with either meiospores or juvenile sporophytes. J Appl Phycol 31:3051–3060
Kohler KE, Gill SM (2006) Coral Point Count with Excel extensions (CPCe): A Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Computers Geosci 32:1259–1269
Løvås SM, Tørum A (2001) Effect of the kelp Laminaria hyperborea upon sand dune erosion and water particle velocities. Coast Eng 44:37–63
Lüning K (1979) Growth strategies of three Laminaria species (Phaeophyceae) inhabiting different depth zones in the sublittoral region of Helgoland (North Sea). Mar Ecol Prog Ser 1:195–207
Mooney-McAuley KM, Edwards MD, Champenois J, Gorman E (2016) Best practice guidelines for seaweed cultivation and analysis. Public output report of the EnAlgae project, Swansea, pp 36
Peteiro C, Sánchez N, Martínez B (2016) Mariculture of the Asian kelp Undaria pinnatifida and the native kelp Saccharina latissima along the Atlantic coast of Southern Europe: an overview. Algal Res 15:9–23
Rasband WS (2012) ImageJ: image processing and analysis in Java. Astrophysics source code library, ascl-1206
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/
RStudio Team (2022) RStudio: integrated development environment for R. RStudio, Inc., Boston. http://www.rstudio.com/
Stévant P, Rebours C, Chapman A (2017) Seaweed aquaculture in Norway: recent industrial developments and future perspectives. Aquac Int 25:1373–1390
Werner A, Clarke D, Kraan S (2004). Strategic review and the feasibility of seaweed aquaculture in Ireland. NDP Marine RTDI Desk Study Series, Marine Institute, Galway 120 pp
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York.https://ggplot2.tidyverse.org
Wilke CO (2020) cowplot: streamlined plot theme and plot annotations for ggplot2. R package v1.1.1: https://CRAN.R-project.org/package=cowplot
World Bank (2023). Global Seaweed: New and Emerging Markets Report, 2023. World bank, Washington, DC Bank. http://hdl.handle.net/10986/40187
Zheng Y, Jin R, Zhang X, Wang Q, Wu J (2019) The considerable environmental benefits of seaweed aquaculture in China. Stock Environ Risk Assess 33:1203–1221
Zuur A, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
This research was supported by the Biomass Feedstocks Innovation Programme (Department for Energy Security and Net Zero). MC acknowledges support from the Natural Environment Research Council under the Queen’s University Belfast and University of Aberdeen Doctoral Research and Training Doctoral Training Partnership (QUADRAT DTP).
Funding
This research was supported by the Biomass Feedstocks Innovation Programme (Department for Energy Security and Net Zero).
Author information
Authors and Affiliations
Contributions
LK conceptualised the study. LSW, EH, MC and LK conducted the hatchery experiments. EMC and LSW performed the data analysis and visualisation. All authors contributed to the critical revision and final approval of the manuscript which was led by EMC and LK.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cunningham, E.M., Wright, L.S., Crowe, M. et al. Under pressure: inhibited sporophyte growth of the sugar kelp Saccharina latissima (Phaeophyceae). J Appl Phycol (2024). https://doi.org/10.1007/s10811-024-03347-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10811-024-03347-2