Abstract
Seaweed cultivation, including kelp species, is rapidly expanding in many regions. A widely assumed co-benefit of seaweed farming is increased local carbon sequestration rates (thereby contributing to climate change mitigation), although direct field-based measurements of carbon assimilation and release are largely lacking. We quantified growth, erosion and dislodgement rates of farmed Saccharina latissima in Porthallow Bay (Cornwall, UK) throughout a typical cultivation season to provide insights into the carbon sequestration potential of small-scale kelp farms. Blade elongation rates increased from ~ 1.3 cm day−1 to ~ 2.3 cm day−1 in March–April, before declining to 1.4 cm day−1 by May. Meanwhile, erosion rates remained low, ranging from ~ 0.5 to ~ 0.8 cm day−1. Dislodgement rates decreased from 20% of plants in January–February to 5% in April–May. Rates of carbon accumulation and loss increased from January to May, related to an increase in standing stock. Conservative first-order estimates suggest that the farm captures 0.14 t C ha−1 y−1, of which up to 70% is released into the environment as particulate organic carbon. Based on previous estimates of carbon burial and storage rates, the farm may sequester 0.05 t CO2e ha−1 y−1. These values suggest that scaling-up European kelp farming should be motivated by other co-benefits, such as low-carbon product alternatives, job creation and potential biodiversity gains, and not be solely driven by a perceived meaningful increase in carbon sequestration. Importantly, further information needs to be obtained from a variety of cultivation sites to develop a better understanding of carbon dynamics associated with kelp farms.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
In 2009 the term Blue Carbon was coined to highlight the importance of the ocean and its ecosystems as global carbon sinks (Nellemann et al. 2009). In particular, salt marshes, mangrove forests and seagrass meadows, were identified as key carbon sinks due to their capacity to accrete allochthonous and autochthonous carbon within their associated sediments (Duarte et al. 2013). Initially, macroalgae ecosystems such as kelp forests and fucoid beds were excluded from Blue Carbon frameworks as most do not accrete carbon locally, due to the rocky substrates they are often associated with, but rather export carbon as detritus (Hill et al. 2015). However, recent studies have argued for the inclusion of natural seaweed habitats because of their high rates of productivity and detrital export, considerable spatial extent, and their function as carbon donors in coastal ecosystems (Krause-Jensen and Duarte 2016; Pessarrodona et al. 2018; Duarte et al. 2022; Filbee-Dexter et al. 2022; Pessarrodona et al. 2022; Smale et al. 2022). As such, these ecosystems provide substantial nature-based solutions for curbing the rate of global warming (United Nations Environment Programme 2023). This means that, alongside reductions in greenhouse gas emissions, the restoration and protection of natural carbon sinks has been identified as an important component of mitigating anthropogenic climate change (IPCC 2022). Concurrently, the seaweed farming industry is expanding rapidly across the globe to meet the increasing demand for seaweed-derived products (FAO 2020). Carbon sequestration from seaweed farming is being increasingly proposed as a co-benefit that may contribute to climate change mitigation, although direct measurements of carbon uptake and release remain lacking which presents issues for decision-making around licenses, community engagement and developing carbon credit markets (Pessarrodona et al. 2024).
With regards to climate change mitigation, some representatives from industry, NGOs and academia have proposed large-scale cultivation of seaweeds (i.e. offshore afforestation) specifically for sinking biomass to the deep ocean to increase sequestration rates (as recently evaluated by Ould and Caldwell 2022; Ross et al. 2022)). However, the general consensus is that the evidence base, technological developments and ethical considerations do not support such an approach (Boyd et al. 2022; Ricart et al. 2022; Ross et al. 2022). Rather, where seaweed is grown for other purposes (e.g., human food, alginates, low-carbon products), some biomass is passively released into marine environment during the cultivation process. For cultivated kelp species, biomass may be released via chronic erosion, occurring mostly at the distal tips of the blades, and/or the dislodgement of whole (or partial) plants (Pedersen et al. 2020). Dislodgement and erosion rates are positively correlated with, often seasonal, increases in temperature and low nitrate levels, which lead to tissue degradation and therefore an increased likelihood of breakage (Rothäusler et al. 2009; Krumhansl and Scheibling 2012; Yorke et al. 2013). Heavy wave action associated with storm activity can also lead to high rates of dislodgement (Smale and Vance 2016). Meanwhile, epiphytic loading by bryozoans and other taxa can cause extensive defoliation, limit nutrient uptake by kelp and make tissue more brittle, again increasing susceptibility to breakage and erosion (Hurd et al. 1994, 2000; Krumhansl and Scheibling 2011). Grazing wounds, superficial excavations and perforations, also increase kelp erosion and dislodgement as weakened tissue becomes more vulnerable to breakage (Krumhansl and Sheibling 2011). The combination of epiphyte and grazer pressure can cause high rates of erosion and biomass release into the environment (Krumhansl and Scheibling 2011). As such, to optimise crop yield and quality, cultivated biomass is harvested before the onset of destructive biofouling and plant senescence (Wilding et al. 2021).
Providing that kelp-derived carbon lost via erosion and dislodgement is not consumed, degraded or remineralised, it has the potential to be sequestered if transferred to and stored within carbon sink habitats (Krause-Jensen and Duarte 2016). To mitigate climate change and to be recognised as ‘additional’ carbon in crediting schemes, this carbon needs to be buried for geologically relevant timescales (> 100 years) (GESAMP 2019; WWF 2019). For natural seaweed ecosystems, Krause-Jensen & Duarte (2016) estimated that ~ 15% of particulate organic carbon (POC) exported from algal habitats is sequestered in either the deep sea and continental shelf sediments combined. Under farm conditions, the release of seaweed biomass (and therefore the amount of carbon potentially available for sequestration) is minimised to optimise product yield and quality (Fieler et al. 2021). However, different farming practices, from hatchery to harvest, affect the amount of biomass released into the environment and therefore potentially available for carbon sequestration (Forbord et al. 2020; Boderskov et al. 2021; Fieler et al. 2021; Matsson et al. 2021).
Estimates of the carbon sequestration potential of seaweed farming are highly variable and geographically biased, with the majority of studies originating from Asia (Duarte et al., preprint). In general, estimates of carbon sequestration potential have been derived from rates of primary production and carbon tissue content, although a recent study showed that the amount of carbon held within sediments directly beneath seaweed farms was greater than within reference sediments (Duarte et al., preprint). In 2021, Asia was responsible for ~ 99.6% of global seaweed aquaculture production, whilst the contribution from Europe was negligible, at ~ 0.1% (FAO 2023), indicative of the potential for growth of the seaweed industry across the region (Araújo et al. 2021). Seaweed farming in Europe is a rapidly growing industry driven by, in part, a prospective climate change mitigation strategy focussed on blue carbon. In light of geographical biases, the poor knowledge base underpinning blue carbon science and the emerging European industry, it is critical to address pressing knowledge gaps and to provide context-specific evidence. Here, we quantified carbon accumulation and loss from Saccharina latissima (sugar kelp), which is one of the most intensively farmed seaweed species in the north Atlantic and Pacific, due to its high growth rates and relative ease of culturing (Sæther et al. 2024) We examined carbon dynamics by monitoring growth, erosion and dislodgement rates through a growing season at a typical, small-scale low-trophic aquaculture site in the southwest of the UK. Such field-based measurements are needed to inform the carbon sequestration potential of kelp farming in Europe and thereby its contribution to climate change mitigation.
Material and methods
Study site
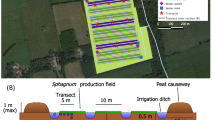
The study focused on cultivated Saccharina latissima (sugar kelp) and was conducted in Porthallow Bay, Cornwall, UK (50◦04’ N, 5◦04’ W) (Fig. 1A). The Bay is open and exposed to east and south-easterly fetch from the English Channel, although the Lizard Peninsula provides shelter from the prevailing south-westerly winds and swells from the Atlantic Ocean (see Fig. 1A). The farm site is situated ~ 500 m offshore, with multiple ~ 200 m header lines suspended ~ 1 m below the surface (Fig. 1B). Mytilus edulis (blue mussels) are predominantly farmed at the site (total farm footprint of ~ 16 ha), with ~ 2 ha of sugar kelp cultivated on dropper lines attached to pre-existing header lines (Fig. 1C). Beneath the farm, water depths vary from 5–15 m, with a ~ 5 m tidal range, and the seabed is comprised of a mix of rocky substrate, soft sediment and maerl gravel. In November 2022, twine seeded with sugar kelp sporophytes (material provided by Hortimare, Netherlands) was wound onto 200 braided dropper lines and then deployed across 2 ha of farm infrastructure. Cultivated kelp was then monitored for 5 months across the growing season (January-May 2023).
Environmental data
To quantify key environmental variables during the experimental period, HOBO pendant loggers (64 k temperature/light sensors; UA-002–64) were deployed ~ 1 m below the water surface (i.e. at a comparable depth to the header lines) within the seaweed farm to measure temperature (°C) and light intensity (lux) every 15 min. Photoperiod was extracted for the same sampling period, at the appropriate latitude, using the R package meteor (Hijmans et al. 2023).
Experimental set-up
At the start of each month of observation, 5 ‘mature’ plants (total length > 15 cm) within a 1 m marked section of a labelled dropper line were tagged using a combination of cable-tie and surgical tubing around the stipe. We focused on ‘mature’ plants as the vast majority of individuals were from the initial cohort of seeded plants, being similar in length and development time, although some ‘sub-canopy’ small plants were also present. Using a modified version of the hole-punch method, holes were excised at 10 and 15 cm from the base of each blade (Parke 1948)—the second hole was used to identify growth outside of the meristematic tissue area – and the length of the blade measured and recorded. At the end of the month (see Table S1 for exact sampling intervals), the position of the holes relative to the base and distal tip of the blade was recorded to estimate rates of elongation and loss of material (see Fig. S1). Dislodgement was determined from the number of lost tagged plants each month. Moreover, the density of mature plants was quantified by enumerating individuals within a 20 cm section of the dropper line. This approach to measuring growth, erosion and dislodgement was repeated on four dropper lines distributed across the seaweed cultivation area (n = 20 tagged plants per month).
At the end of each month, tagged kelp were retrieved and returned to the laboratory for further biometric analysis. Subsequently, new plants were selected (5 plants on each of 4 droppers), tagged and hole-punched to capture growth, erosion and dislodgement rates for the following month. On return to the laboratory, three sections of blade tissue (basal, mid and distal, 5 cm in length) as well as the stipe-holdfast complex were excised from each plant and weighed to obtain fresh weight (FW). Samples were then dried (at 60 °C for 48 h) and reweighed to obtain dry weight (DW) values, the average ratio of FW to DW was then calculated for each month (Table S2).
Conversion to carbon dynamics, scaling-up and statistical analysis
In cultivated kelp, biomass accumulation primarily occurs through both elongation of the blade and allocation of biomass to the stipe-holdfast complex. In natural kelp populations, biomass accumulation also occurs via a ‘thickening’ of blade tissues as sugars and other storage compounds are laid down throughout summer in advance of the following winter/spring growth season (Bartsch et al. 2008). In the cultivation context, plants are generally harvested whilst still in the fast-growth phase and so this process of biomass accumulation via blade thickening was considered to be negligible and not directly measured here. Biomass accumulation related to blade elongation was calculated by converting the daily elongation rate to a biomass value obtained from the basal segment of the blade tissue. Biomass accumulation related to the stipe-holdfast complex was estimated by calculating the difference in average biomass of this structure between consecutive months. Biomass loss via erosion was estimated by converting the daily loss rate of tissue to a biomass value obtained from the distal segment of the blade tissue. Rates of biomass accumulation (via blade elongation and stipe-holdfast growth) and biomass loss (via erosion) were multiplied by the density of plants per dropper to yield a process rate per month. Dislodgement rates were converted to biomass release values by multiplying average plant biomass each month by the number of plants lost (i.e. dislodgement rate x density). The FW:DW described above and the carbon content of sugar kelp derived from the literature (29.75%; Brady-Campbell et al. 1984; Gevaert et al. 2001) was then applied to elongation, growth, erosion, dislodgment and biomass values to estimate carbon accumulation and loss rates. Conservative first-order estimates of the amount of carbon captured and released by the farm per hectare (t C ha−1 y−1) were calculated using the monthly averages of dropper carbon accumulation and loss rates multiplied by the number of droppers across the farm (200) which corresponded to its size (2 ha). Processes are presented as annual rates although in actuality the growth season of sugar kelp is ~ 6 months and for the remaining half-year seaweed biomass is currently not cultivated.
We formally examined statistical differences between droppers and months using univariate permutational analysis of variance (PERMANOVA), which was carried out in PRIMER v7 using the PERMANOVA add-on (Clarke and Gorley 2015). For response variables measured at the plant level (i.e. elongation and erosion rates, plant biomass) we used a full model with ‘month’ as a fixed factor and ‘dropper’ as a random nested factor, to examine variability across the farm. Where response variables were calculated at the dropper level (i.e., stipe-holdfast growth, dislodgement, density, total carbon accumulation and loss) we used a simpler model with ‘month’ as a single fixed factor. In all cases, permutations (9999 under an unrestricted model) were based on Euclidean distances between untransformed data. Monte Carlo simulations were used when permutations were low to determine significance to mitigate the effect of small sample sizes. Where a significant effect of the fixed factor was detected (p < 0.05), pairwise comparisons were conducted to determine which months differed from one another. Where it was not possible to collect monthly data due to adverse weather conditions, an estimate was derived from the means of the month prior and following. These estimates were excluded from statistical analyses but included in figures and descriptions of trends. All summary statistics were carried out in R v4.3.1 using the package dplyr (R Core Team 2023; Wickham et al. 2023).
Results
Environmental data
Environmental data collected from 1 m below the water surface at the seaweed farm in Porthallow Bay, showed that average daily (07:00–19:00) irradiance increased from 9.65 µmol photons m−2 s−1 in January to its peak in May at 312.73 µmol photons m−2 s−1 (Fig. 2A). Photoperiod increased from ~ 8 h in January to ~ 16 h in May (Fig. 2A). Average daily sea temperatures remained stable at around 10 °C from January to April, and optimal temperatures for plant growth (< 15 °C) were not exceeded until June, by which point sampling had concluded (Fig. 2B).
Rates of biomass growth and loss
At the start of the monitoring period, sugar kelp individuals were ~ 41 cm in length and grew, in terms of blade elongation, at an average rate of 1.26 ± 0.35 cm day−1 (Fig. 3A). Compared to January–February, growth rates increased ~ 1.5 and ~ 1.8 fold in February–March and March–April, respectively. By April–May, growth rates began to decline. Growth rates differed significantly between months, with no effect of dropper line (Table S3). The average growth, recorded as fresh weight biomass accumulation, of the holdfast-stipe complex almost quadrupled from January (0.028 ± 0.002 g FW day−1) to April (0.076 ± 0.037 g FW day−1) (Fig. 3B). By April–May, growth declined to 0.009 ± 0.022 g FW day−1, slower than the initial rates recorded in January–February. While there was an overall significant difference between months, variability increased throughout the monitoring period, and growth of the stipe-holdfast complex did not differ between March and April (Fig. 3B; Table S4).
The monthly A) blade elongation, B) holdfast-stipe (Hf-St) growth, C) erosion rates of cultivated S. latissima, D) percentage dislodgement, E) biomass of plants and F) density per 20 cm of cultivation line. The box plots show medians, 1st and 3rd quartiles, with whiskers showing maximum and minimum values, and dots representing outliers. Diamonds indicate mean values, with dashed-line diamonds representing the modelled mean for missing months (see method for calculation), whilst ‘na’ indicates unavailable data. The level of significance is indicated with asterisks, where ‘*’ is p < 0.05, ‘**’ is p < 0.01 and ‘***’ is p < 0.001 and significance between months is denoted by different lettering
Whilst average growth (elongation and holdfast-stipe complex growth) slowed in April–May, mean erosion peaked here at 0.80 ± 0.91 cm day−1 (Fig. 3C). Throughout the growing season erosion were consistently low, ranging from 0.48 ± 0.37 to 0.55 ± 0.36 cm day−1, with no significant difference identified between months (Table S3). Similarly, there was no significant difference in percentage dislodgement between months, with values highly variable throughout (Table S4). Nevertheless, a trend of decreasing dislodgement can be observed throughout the growing season, with average loss of ~ 20% in January–February and ~ 5% in April–May (Fig. 3D).
Biomass and density
Individual plant biomass increased significantly over the growing season, from an average 35.9 ± 12.4 g to 187.0 ± 150.0 g (Fig. 3E; Table S3). Similarly, a significant increase in plant density per 20 cm of dropper line was observed, from 8.67 ± 1.15 to 54.8 ± 10.9 mature plants (Fig. 3F; Table S4).
Carbon accumulation and loss
Using monthly average biomass and density data, standing stock (i.e. biomass × density) in terms of carbon was calculated for a dropper line. We recorded an increase from ~ 0.48 kg C dropper−1 in February–March to ~ 2.9 kg C dropper−1 in April–May (Fig. 4A). Carbon accumulation average, relating to blade elongation, increased steadily from 0.92 ± 0.25 g C day−1 dropper−1 in January–February and peaked at 14.90 ± 5.47 g C day−1 dropper−1 in April–May (Fig. 4B). Carbon accumulation via elongation was significantly different between months, although post-hoc comparisons showed that March–April and April–May were not significantly different from each other (Table S5). Carbon accumulation average, in terms of the holdfast-stipe complex, were initially low at 0.06 ± 0.01 g C day−1 dropper−1 (Fig. 4C). Accumulation average continued to increase to a peak of 0.66 ± 0.32 g C day−1 dropper−1 in March–April, after which they declined to 0.13 ± 0.34 g C day−1 dropper−1 in April–May. Here, rates differed significantly among months, except between March–April and April–May (Table S5).
The A) standing stock of carbon per dropper of cultivated S. latissima from January – May 2023, shown with carbon accumulation rates for B) blade elongation and C) holdfast-stipe (Hf-St) growth, carbon loss rates via D) erosion and E) dislodgement. The box plots show medians, 1st and 3rd quartiles, with whiskers showing maximum and minimum values, and dots representing outliers. Diamonds indicate mean values, with dashed-line diamonds representing the modelled mean for missing months (see method for calculation), whilst ‘na’ indicates unavailable data. The level of significance is indicated with asterisks, where ‘*’ is p < 0.05, ‘**’ is p < 0.01 and ‘***’ is p < 0.001 and significance between months is denoted by different lettering
The average loss of carbon via chronic distal erosion was 0.26 ± 0.18 g C day−1 dropper−1 at the beginning of the monitoring period (Fig. 4D). Like carbon accumulation via elongation, this increased gradually to its peak, 7.40 ± 8.70 g C day−1 dropper−1, in April–May (Fig. 4D). Here, monthly rates were significantly different (Table S5). Carbon lost via dislodgement was highly variable across the growing season, with no significant difference between months (Table S5). The average rate of loss in February–March was 2.87 ± 3.67 g C day−1 dropper−1, almost doubling by April–May (Fig. 4E).
Annual accumulation and loss of carbon
Using conservative first-order estimates, farm-wide carbon accumulation and loss were calculated (Table 1). This estimate suggests that ~ 0.14 t C ha−1 y−1 are accumulated within the biomass of the sugar kelp farmed in Porthallow Bay, of which ~ 70% is lost, almost equally accounted for by erosion and dislodgement.
Discussion
Our field-based measurements of carbon accumulation and release at a small-scale commercial cultivation site informed representative estimates of the carbon sequestration potential of kelp farming. Biomass accumulation by cultivated sugar kelp at Porthallow Bay captured ~ 0.14 t C ha−1 y−1, with ~ 0.09 t C ha−1 y−1 released into the marine environment as detritus (i.e. POC). The proportion of carbon accumulated by farmed kelp subsequently released into the marine environment at the cultivation site (i.e. ~ 70%) was lower compared to both natural kelp beds (~ 82%) (Krumhansl and Scheibling 2012) and a recent comparable estimate for a sugar kelp farm in Northern Ireland (~ 84% NPP) (Dolliver and O’Connor 2022). With regards to natural kelp beds, the greater relative rates of carbon release may be related to the highly dynamic, shallow, wave and tide-exposed conditions that often characterise these habitats (Smale et al. 2022). The reasons for the higher rates of release at the farm site in Northern Ireland remain unclear but may include differences in tidal dynamics, biofouling, nutrient availability or seeding and monitoring techniques (Dolliver and O’Connor 2022). Based on these conservative first-order estimates, carbon capture and release at the farm in Porthallow Bay falls within the range reported across other European studies, which are generally lower than those reported in Asia (Table 2). By applying a previous estimate of ~ 15% of seaweed-derived POC that is released into the environment being sequestered (Krause-Jensen and Duarte 2016), we estimated a sequestration rate of ~ 0.014 t C ha−1 y−1 (71.98 g C m dropper−1 y−1) for the farm in Cornwall, southwest UK. Despite this relatively low sequestration rate, this study provides a starting point for predicting the carbon sequestration potential of a scaled-up seaweed farming industry. With 2,900,000 ha suitable for farming sugar kelp and Laminaria digitata (oarweed), in English waters alone (MMO 2019), the maximum hypothetical carbon sequestration potential is estimated at 40,600 t C y−1, or 149,002 t CO2e. Clearly, this potential sequestration rate does not take in to account the carbon emissions needed to establish and maintain farm infrastructure, nor to seed and harvest the seaweed lines, which would be needed for a full ‘forensic’ account of the net carbon gains. Rather, this is an estimate of the additional potential carbon co-benefit derived from pre-existing, operational farm sites.
Our study highlighted pronounced seasonality in core processes. Throughout the growing season, the light and temperature environment varied markedly, although conditions recorded remained favourable for growth of sugar kelp (Fortes and Lüning 1980; Peteiro et al. 2016). Growth rates related to both blade elongation and holdfast-stipe growth increased markedly from winter (i.e. January) to spring (i.e. March), reflecting seasonal patterns of growth observed for natural populations in the northeast Atlantic (Parke 1948; Sjøtun 1993). After March growth rates slowed significantly, which may be related to reduced nutrient availability, responses to photoperiod or endogenous circannual growth rhythms (Bartsch et al. 2008; Peteiro et al. 2016). In terms of release of biomass, erosion rates were consistently low throughout the growing season. This highlights the chronic nature of distal erosion, however, this is expected to increase as temperatures and biofouling increase, and nutrient availability decreases, which normally coincides with the end of the growing season (Krumhansl and Scheibling 2011, 2012). As such, chronic erosion of the distal tips of blades may represent a relatively unimportant mechanism of detritus release through the growing season in this case, but would be expected to increase in relative importance as temperatures and biofouling and grazer pressure increase through summer. Extending the monitoring period past harvest (April–May) would likely capture this pattern.
The decrease in relative dislodgement rates throughout the growing season could be attributed to larger plants, indicated by the increase in biomass throughout the same period, being more resistant to breakage or removal by wave action than the smaller, more fragile sporophytes present in January. This is likely related to greater holdfast size and attachment strength as well as reduced storminess from winter through to spring/summer. Like biomass, plant density increased from January to May. This, in combination with decreasing dislodgement, resulted in more densely populated lines of larger sugar kelp plants by May. Like erosion, dislodgement is expected to increase with the onset of warmer temperatures, reduced nutrient availability and increased biofouling causing kelp to become more brittle and prone to breakage (Rothäusler et al. 2009; Krumhansl and Scheibling 2012; Yorke et al. 2013). This pattern could again be captured by extending the monitoring period and delaying harvest. Postponing harvest would likely increase the amount of dislodgment and erosion-derived POC released into the marine environment. However, extending the growing season would reduce the quality and quantity of harvestable, marketable biomass for which the seaweed is primarily grown, not the additional, passive co-benefit of carbon sequestration (Fieler et al. 2021).
Whilst these results show that kelp farming captures carbon, the associated sequestration potential is relatively low and farming techniques and harvesting schedules are not optimised with carbon capture in mind. Therefore, carbon capture should be viewed as a passive co-benefit of kelp farming rather than a major driver. Instead, the focus should be directed towards other benefits associated with low trophic marine aquaculture that support climate change mitigation to a greater degree, like future food security, bioenergy production and the replacement of high-carbon footprint products (Krause et al. 2022; O’Dell et al. 2023; Troell et al. 2023). Importantly, the loss of kelp-derived carbon as detrital POC, as presented here, is just one pathway by which carbon can be exported and potentially sequestered. To achieve a more holistic understanding of the carbon sequestration potential of kelp farming, other processes and pathways need to be considered, such as the release and fate of dissolved organic carbon (DOC), the transport and persistence of kelp-derived carbon in marine environments, rates of long-term burial and storage, and the flux of carbon dioxide between the atmosphere and sea (Hurd et al. 2022, 2023). Indeed, a major limitation of the current study was the application of an assumed sequestration rate (~ 15% of exported carbon, as proposed by Krause-Jense and Duarte 2016), rather than applying direct measurements of carbon burial and long-term sequestration within the study region. Further work should focus on addressing the current uncertainty around these processes. We also recommend conducting similar surveys across a range of farm sites in the UK and Europe that encompass different environmental contexts, both monoculture and co-culture of seaweeds, and diverse cultivation practices to build a more robust, context-specific evidence base. With seaweed farming in its infancy in Europe, there is an opportunity to collate carbon capture and storage estimates using standardised methodologies, as has been done elsewhere (Duarte et al., preprint 2023). This would support the credible and measurable growth of the sector while upholding transparency regarding potential benefits. Additionally, further research should focus on conducting life cycle assessments (LCAs) on functional kelp farms to highlight where emissions can be avoided through process optimization and by replacing carbon-intensive products with low-carbon, kelp-derived products (Duarte et al. 2021). This is important for ensuring the sustainable development of the seaweed industry in Europe, as well as identifying the climate change mitigation potential of the sector and products outside of the growing season.
In conclusion, we recorded high relative rates of kelp-derived POC release, with ~ 70% of total biomass accumulation being exported to the marine environment. However, when assessed as the absolute amount of carbon likely to be locally stored, based on an estimated sequestration rate and the scale and stocking density of the cultivation site, the carbon sequestration potential of UK seaweed farming is relatively low. Even so, small-scale low trophic aquaculture may have multiple ‘stacked benefits’, including habitat provisioning and biodiversity maintenance (Corrigan et al. 2022, 2023) as well as additional carbon accumulation and release, which need to be considered holistically to reliably assess the risks and benefits associated with continued expansion of the seaweed industry. In the UK, natural kelp habitats extend across much of the shallow rocky coastline (Smale et al. 2013), where they exhibit high rates of primary productivity (Pessarrodona et al. 2018; Smale et al. 2020) and support elevated biodiversity (Teagle et al. 2018; Bué et al. 2020; King et al. 2021). The amount of carbon released into the marine environment via natural kelp populations will likely be several orders of magnitude greater than farmed kelp, even if scaled-up, and understanding and protecting carbon stocks and flows through these critical ecosystems should therefore remain a priority (Smale et al. 2018; Queirós et al. 2023).
Data Availability
Data will be made available on request.
Code availability
Code will be made available on request.
References
Araújo R, Vázquez Calderón F, Sánchez López J, Azevedo IC, Bruhn A, Fluch S, Garcia Tasende M, Ghaderiardakani F, Ilmjärv T, Laurans M, Mac Monagail M, Mangini S, Peteiro C, Rebours C, Stefansson T, Ullmann J (2021) Current status of the algae production industry in Europe: an emerging sector of the Blue Bioeconomy. Front Mar Sci 7:626389
Bartsch I, Wiencke C, Bischof K, Buchholz CM, Buck BH, Eggert A, Feuerpfeil P, Hanelt D, Jacobsen S, Karez R, Karsten U, Molis M, Roleda MY, Schubert H, Schumann R, Valentin K, Weinberger F, Wiese J (2008) The genus Laminaria sensu lato: recent insights and developments. Eur J Phycol 43:1–86
Boderskov T, Nielsen MM, Rasmussen MB, Balsby TJS, Macleod A, Holdt SL, Sloth JJ, Bruhn A (2021) Effects of seeding method, timing and site selection on the production and quality of sugar kelp, Saccharina latissima: A Danish case study. Algal Res 53:102160
Boderskov T, Rasmussen MB, Bruhn A (2023) Upscaling cultivation of Saccharina latissima on net or line systems; comparing biomass yields and nutrient extraction potentials. Front Mar Sci 10:992179
Boyd PW, Bach LT, Hurd CL, Paine E, Raven JA, Tamsitt V (2022) Potential negative effects of ocean afforestation on offshore ecosystems. Nat Ecol Evol 6:675–683
Brady-Campbell MM, Campbell DB, Harlin MM (1984) Productivity of kelp (Laminaria spp.) near the southern limit in the Northwestern Atlantic Ocean. Mar Ecol Prog Ser 18:79–88
Bué M, Smale DA, Natanni G, Marshall H, Moore PJ (2020) Multiple-scale interactions structure macroinvertebrate assemblages associated with kelp understory algae. Divers Distrib 26:1551–1565
Chung IK, Oak JH, Lee JA, Shin JA, Kim JG, Park K-S (2013) Installing kelp forests/seaweed beds for mitigation and adaptation against global warming: Korean project overview. ICES J Mar Sci 70:1038–1044
Clarke KR, Gorley RN (2015) Getting started with PRIMER V7. PRIMER-E Ltd, Plymouth
Corrigan S, Brown AR, Ashton IGC, Smale DA, Tyler CR (2022) Quantifying habitat provisioning at macroalgal cultivation sites. Rev Aquac 14:1671–1694
Corrigan S, Brown AR, Tyler CR, Wilding C, Daniels C, Ashton IGC, Smale DA (2023) Development and diversity of epibiont assemblages on cultivated Sugar Kelp (Saccharina latissima) in relation to farming schedules and harvesting techniques. Life 13:209
Corrigan S, Brown AR, Tyler CR, Wilding C, Daniels C, Ashton IGC, Smale DA (2024) Home sweet home: Comparison of epibiont assemblages associated with cultivated and wild sugar kelp (Saccharina latissima), co-cultivated blue mussels (Mytilus edulis) and farm infrastructure. J Appl Phycol 38:611–625
Dolliver J, O’Connor NE (2022) Estimating growth, loss and potential carbon sequestration of farmed kelp: a case study of Saccharinalatissima at Strangford Lough Northern Ireland. Appl Phycol 3:324–339
Duarte CM, Losada IJ, Hendriks IE, Mazarrasa I, Marbà N (2013) The role of coastal plant communities for climate change mitigation and adaptation. Nat Clim Chang 3:961–968
Duarte CM, Bruhn A, Krause-Jensen D (2021) A seaweed aquaculture imperative to meet global sustainability targets. Nat Sustain 5:185–193
Duarte CM, Gattuso J-P, Hancke K, Gundersen H, Filbee-Dexter K, Pedersen MF, Middelburg JJ, Burrows MT, Krumhansl KA, Wernberg T, Moore P, Pessarrodona A, Ørberg SB, Pinto IS, Assis J, Queirós AM, Smale DA, Bekkby T, Serrão EA, Krause-Jensen D (2022) Global estimates of the extent and production of macroalgal forests. Glob Ecol Biogeogr 31:1422–1439
Duarte CM, Delgado-Huertas A, Marti E, Gasser B, San Martin I et al (2023) Carbon burial in sediments below seaweed farms. bioRXiv. https://doi.org/10.1101/2023.01.02.522332
FAO (2020) The State of World Fisheries and Aquaculture 2020. FAO, Rome
FAO (2023) Global aquaculture production quantity (1950–2021). FAO, Rome
Fieler R, Greenacre M, Matsson S, Neves L, Forbord S, Hancke K (2021) Erosion dynamics of cultivated kelp, Saccharina latissima, and implications for environmental management and carbon sequestration. Front Mar Sci 8:632725
Filbee-Dexter K, Pessarrodona A, Duarte CM, Krause-Jensen D, Hancke K, Smale DA, Wernberg T (2023) Seaweed forests are carbon sinks that can mitigate CO2 emissions: a comment on Gallagher et al. (2022). ICES J Mar Sci 80:1814–1819
Forbord S, Steinhovden KB, Solvang T, Handå A, Skjermo J (2020) Effect of seeding methods and hatchery periods on sea cultivation of Saccharina latissima (Phaeophyceae): a Norwegian case study. J Appl Phycol 32:2201–2212
Fortes MD, Lüning K (1980) Growth rates of North Sea macroalgae in relation to temperature, irradiance and photoperiod. Helgol Meeresunters 34:15–29
Gao G, Gao L, Jiang M, Jian A, He L (2022) The potential of seaweed cultivation to achieve carbon neutrality and mitigate deoxygenation and eutrophication. Environ Res Letts 17:014018
GESAMP (2019) High level review of a wide range of proposed marine geoengineering techniques. GESAMP Report 98
Gevaert F, Davoult D, Creach A, Kling R, Janquin M-A, Seuront L, Lemoine Y (2001) Carbon and nitrogen content of Laminaria saccharina in the eastern English Channel: biometrics and seasonal variations. J Mar Biol Assoc U K 81:727–734
Hijmans RJ, Nelson G, Waterloo M (2023) meteor: Meteorological Data Manipulation. CRAN. https://cran.r-project.org/web/packages/meteor/meteor.pdf
Hill R, Bellgrove A, Macreadie PI, Petrou K, Beardall J, Steven A, Ralph PJ (2015) Can macroalgae contribute to blue carbon? An Australian Perspective Limnol Oceanogr 60:1689–1706
Hurd CL, Durante KM, Chia F-S, Harrison PJ (1994) Effect of bryozoan colonization on inorganic nitrogen acquisition by the kelps Agarurn fimbriatum and Macrocystis integrifolia. Mar Biol 121:167–173
Hurd CL, Durante KM, Harrison PJ (2000) Influence of bryozoan colonization on the physiology of the kelp Macrocystis integrifolia (Laminariales, Phaeophyta) from nitrogen-rich and -poor sites in Barkley Sound, British Columbia, Canada. Phycologia 39:435–440
Hurd CL, Gattuso JP, Boyd PW (2023) Air-sea carbon dioxide equilibrium: Will it be possible to use seaweeds for carbon removal offsets? J Phycol 60:4–14
Hurd CL, Law CS, Bach LT, Britton D, Hovenden M, Paine E, Raven JA, Tamsitt V, Boyd PW (2022) Forensic carbon accounting: Assessing the role of seaweeds for carbon sequestration. J Phycol 58:347–363
IPCC (2022) Climate change 2022: impacts, adaptation and vulnerability. In: Pörtner H-O, Roberts DC, Tignor M, Poloczanska ES, Mintenbeck K, Alegría A, Craig M, Langsdorf S, Löschke S, Möller V, Okem A, Rama B (eds) Contribution of working group II to the sixth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, p 3056
King N, Moore P, Wilding C, Jenkins H, Smale D (2021) Multiscale spatial variability in epibiont assemblage structure associated with stipes of kelp Laminaria hyperborea in the northeast Atlantic. Mar Ecol Prog Ser 672:33–44
Krause G, Le Vay L, Buck BH, Costa-Pierce BA, Dewhurst T, Heasman KG, Nevejan N, Nielsen P, Nielsen KN, Park K, Schupp MF, Thomas J-B, Troell M, Webb J, Wrange AL, Ziegler F, Strand Å (2022) Prospects of low trophic marine aquaculture contributing to food security in a net zero-carbon world. Front Sustain Food Syst 6:875509
Krause-Jensen D, Duarte CM (2016) Substantial role of macroalgae in marine carbon sequestration. Nat Geosci 9:737–742
Krumhansl K, Scheibling R (2012) Production and fate of kelp detritus. Mar Ecol Prog Ser 467:281–302
Krumhansl K, Scheibling R (2011) Detrital production in Nova Scotian kelp beds: patterns and processes. Mar Ecol Prog Ser 421:67–82
Lian Y, Wang R, Zheng J, Chen W, Chang L, Li C, Yim SC (2023) Carbon sequestration assessment and analysis in the whole life cycle of seaweed. Environ Res Lett 18:074013
Matsson S, Metaxas A, Forbord S, Kristiansen S, Handå A, Bluhm BA (2021) Effects of outplanting time on growth, shedding and quality of Saccharina latissima (Phaeophyceae) in its northern distribution range. J Appl Phycol 33:2415–2431
MMO (2019) Identification of areas of aquaculture potential in English waters (MMO 1184). Marine Management Organisation, Newcastle upon Tyne, p 22
Nellemann C, Corcoran E, Duarte CM, Valdes L, De Young C, Fonseca LE, Grimsditch G (eds) (2009) Blue carbon: the role of healthy oceans in binding carbon. UN Environment Programme, GRID-Arendal, p 78
O’Dell A, Adrian A, Canvin M, De Bettignies F, Filbee-Dexter K, Grisenthwaite R, Hughes A, Hancke K, Krause-Jensen D, Macleod A, Moore P, Ricart A, Rostan J, Stanley M, Smale D, Smeaton C, Troell M, Burrows MT (2023) BLUE FORESTS A review of carbon offset strategies with seaweed aquaculture-feasibility, current knowledge, and suggestions for future research. The Scottish Association for Marine Science, Oban, UK. Report prepared for the Scottish Government
Ould E, Caldwell GS (2022) The potential of seaweed for carbon capture. CABI Reviews 17:009
Parke M (1948) Studies on British Laminariaceae. I. Growth in Laminaria saccharina (L.) Lamour. J Mar Biol Assoc UK 27:651–709
Pedersen MF, Filbee-Dexter K, Norderhaug KM, Fredriksen S, Frisk NL, Fagerli CW, Wernberg T (2020) Detrital carbon production and export in high latitude kelp forests. Oecologia 192:227–239
Pessarrodona A, Assis J, Filbee-Dexter K, Burrows MT, Gattuso J-P, Duarte CM, Krause-Jensen D, Moore PJ, Smale DA, Wernberg T (2022) Global seaweed productivity. Sci Adv 8:eabn2465
Pessarrodona A, Howard J, Pidgeon E, Wernberg T, Filbee-Dexter K (2024) Carbon removal and climate change mitigation by seaweed farming: A state of knowledge review. Sci Total Environ 918:170525
Pessarrodona A, Moore PJ, Sayer MDJ, Smale DA (2018) Carbon assimilation and transfer through kelp forests in the NE Atlantic is diminished under a warmer ocean climate. Glob Chang Biol 24:4386–4398
Peteiro C, Sánchez N, Martínez B (2016) Mariculture of the Asian kelp Undaria pinnatifida and the native kelp Saccharina latissima along the Atlantic coast of Southern Europe: An overview. Algal Res 15:9–23
Queirós AM, Tait K, Clark JR, Bedington M, Pascoe C, Torres R, Somerfield PJ, Smale DA (2023) Identifying and protecting macroalgae detritus sinks toward climate change mitigation. Ecol Appl 33:e2798
R Core Team (2023) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org
Ricart AM, Krause-Jensen D, Hancke K, Price NN, Masqué P, Duarte CM (2022) Sinking seaweed in the deep ocean for carbon neutrality is ahead of science and beyond the ethics. Environ Res Lett 17:081003
Ross F, Tarbuck P, Macreadie PI (2022) Seaweed afforestation at large-scales exclusively for carbon sequestration: critical assessment of risks, viability and the state of knowledge. Front Mar Sci 9:1015612
Rothäusler E, Gómez I, Hinojosa IA, Karsten U, Tala F, Thiel M (2009) Effect of temperature and grazing on growth and reproduction of floating Macrocystis spp. (Phaeophyceae) along a latitudinal gradient. J Phycol 45:547–559
Sæther M, Diehl N, Monteiro C, Li H, Niedzwiedz S, Burgunter-Delamare B, Scheschonk L, Bischof K, Forbord S (2024) The sugar kelp Saccharina latissima II: recent advances in farming and applications. J Appl Phycol 36:1953–1985
Sjøtun K (1993) Seasonal lamina growth in two age groups of Laminaria saccharina (L.) Lamour in Western Norway. Bot Mar 36:433–441
Smale DA, Burrows MT, Moore P, O’Connor N, Hawkins SJ (2013) Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic tlantic perspective. Ecol Evol 3:4016–4038
Smale DA, Moore PJ, Queirós AM, Higgs ND, Burrows MT (2018) Appreciating interconnectivity between habitats is key to blue carbon management. Front Ecol Environ 16:71–73
Smale DA, Pessarrodona A, King N, Burrows MT, Yunnie A, Vance T, Moore P (2020) Environmental factors influencing primary productivity of the forest-forming kelp Laminaria hyperborea in the northeast Atlantic. Sci Rep 10:12161
Smale DA, Pessarrodona A, King N, Moore PJ (2022) Examining the production, export, and immediate fate of kelp detritus on open‐coast subtidal reefs in the Northeast Atlantic. Limnol Oceanogr 67:536–549
Smale DA, Vance T (2016) Climate-driven shifts in species’ distributions may exacerbate the impacts of storm disturbances on North-east Atlantic kelp forests. Mar Freshw Res 67:65–74
Song C, Xiong Y, Jin P, Sun Y, Zhang Q, Ma Z, Gao G (2023) Mariculture structure adjustment to achieve China’s carbon neutrality and mitigate climate change. Sci Total Environ 895:164986
Teagle H, Moore PJ, Jenkins H, Smale DA (2018) Spatial variability in the diversity and structure of faunal assemblages associated with kelp holdfasts (Laminaria hyperborea) in the northeast Atlantic. PLoS ONE 13:e0200411
Troell M, Henriksson PJG, Buschmann AH, Chopin T, Quahe S (2023) Farming the ocean – Seaweeds as a quick fix for the climate? Rev Fish Sci Aquacult 31:285–295
United Nations Environment Programme (2023) Into the Blue: Securing a Sustainable Future For Kelp Forests. UNEP, Nairobi
Visch W, Bergström P, Nylund GM, Peterson M, Pavia H, Lindegarth M (2020) Spatial differences in growth rate and nutrient mitigation of two co-cultivated, extractive species: The blue mussel (Mytilus edulis) and the kelp (Saccharina latissima). Estuar Coast Shelf Sci 246:107019
Wickham H, François R, Henry L, Müller K, Vaughan D (2023) dplyr: A Grammar of Data Manipulation. CRAN. https://cran.r-project.org/web/packages/dplyr/index.html
Wilding C, Tillin H, Corrigan SE, Stuart E, Ashton I, Felstead P, Lubelski A, Burrows M, Smale D (2021) Seaweed aquaculture and mechanical harvesting : an evidence review to support sustainable management. Natural England Research Report NECR378
WWF (2019) WWF position and guidance on voluntary purchases of carbon credits. https://www.worldwildlife.org/publications/wwf-position-and-guidance-on-voluntary-purchases-of-carbon-credits
Yorke C, Miller R, Page H, Reed D (2013) Importance of kelp detritus as a component of suspended particulate organic matter in giant kelp Macrocystis pyrifera forests. Mar Ecol Prog Ser 493:113–125
Zhang X, Boderskov T, Bruhn A, Thomsen M (2022) Blue growth and bioextraction potentials of Danish Saccharina latissima aquaculture — A model of eco-industrial production systems mitigating marine eutrophication and climate change. Algal Res 64:102686
Acknowledgements
We thank the Cornish Seaweed Company and Westcountry Mussels for their assistance with fieldwork and access to the farm. We thank members of the BEECH team who assisted with fieldwork and Simon Walmsley, Tom Brook and Mollie Gupta at WWF-UK for their insightful comments, administrative support and enthusiasm for the project.
Funding
DS was funded by a UKRI Future Leaders Fellowship (MR/X023214/1). MC and other research costs were funded by a WWF-UK contract awarded to PM and DS.
Author information
Authors and Affiliations
Contributions
All authors conceived the idea and designed the surveys. M.C. led fieldwork, sample collection and processing, data analysis and interpretation, with support from D.S. and P.M.. M.C. led manuscript preparation with support from. D.S.. All authors edited and reviewed the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Canvin, M.C., Moore, P.J. & Smale, D.A. Quantifying growth, erosion and dislodgement rates of farmed kelp (Saccharina latissima) to examine the carbon sequestration potential of temperate seaweed farming. J Appl Phycol (2024). https://doi.org/10.1007/s10811-024-03323-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10811-024-03323-w