Abstract
Bisphenol A (BPA) is an emerging organic compound used in the production of epoxy resin, polycarbonate plastics and thermal paper. Following the restrictions on the use of bisphenol A, many substitutes have been produced as its replacement in several consumer products. The main task of this research was to examine the toxic effects of single bisphenol analogues and their mixtures against freshwater microalgae Chlorella vulgaris and Desmodesmus armatus. The findings suggest that bisphenol B, bisphenol C, bisphenol PH (EC50 (14 day): 33.32-43.32 mg L-1) and bisphenol B, bisphenol C, bisphenol FL, bisphenol PH (EC50 (14 day): 30.49-64.54 mg L-1) show strong toxic effects towards C. vulgaris and D. armatus, respectively. In turn, the research results indicate that the toxicity of a mixture of examined bisphenol analogs on both species of green algae is much higher (EC50 (14 day): 24.55-32.68 mg L-1) than the individual toxicity of each component of the mixture. Therefore, it can be concluded that mixtures lead to the occurrence of synergistic effects. The toxicity of the individual bisphenol analogues and their mixture by EC50 (14 day) values in descending order, was as follows: mixture>bisphenol PH> bisphenol B> bisphenol C> bisphenol FL> bisphenol F> bisphenol E for C. vulgaris and bisphenol B> mixture> bisphenol FL> bisphenol C> bisphenol PH> bisphenol E> bisphenol F for D. armatus, respectively. Moreover, the present research expands current knowledge of the ecotoxicological risks of bisphenol analogues to aquatic organisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bisphenol A (BPA) is a high-volume synthetic production substance that has been used since the 1960s as a monomer or additive in the production of epoxy resins, polycarbonate plastics materials and thermal paper (Sirasanagandla et al. 2022). Generally, this compound is used to provide rigidity, strength and resistance of materials applied in the production of many consumer products, including food containers, medical devices, electronic products, dental fillings, toys, water pipes and paints (Durovcova et al. 2022; Xing et al. 2022). In recent years, the demand for BPA production has been steadily increasing and it is estimated that in 2032 it will exceed 12,169 tonnes (Karahalil et al. 2023). Unfortunately, many studies confirmed that BPA has been recognized as a xenoestrogen that mimics the actions of natural oestrogen (17-β estradiol) and negatively affects the endocrine, reproductive, metabolic and nervous systems (Abraham and Chakraborty 2020; Mukhopadhyat et al. 2021; Hamed and Li 2022). In addition to its endocrine-disrupting effects, it can also cause genotoxicity and oxidative stress in aquatic organisms (Czarny-Krzyminska et al. 2023). Due to the high risk of exposure of BPA to humans and other animals, the European Chemical Agency (ECHA) included this xenoestrogen on the Candidate List of Substances of Very High Concern, which contributed to the restriction and prohibition of its industrial use in many applications (ECHA 2017). For example, the usage of BPA in the fabrication of baby feeding bottles has been banned in the European Union, the USA and Canada (Lehmler et al. 2018).
As a result of growing public concern about the toxicity and endocrine activity of BPA, numerous of bisphenol analogues (BPs), such as bisphenol B (BPB), bisphenol C (BPC), bisphenol E (BPE), bisphenol F (BPF), bisphenol FL (BPFL) and bisphenol PH (BPPH) have been exploited as its alternatives in various consumer products. references presents the main physicochemical characteristics and predicted toxic effect of selected bisphenol analogues. (Table 1). For example, BPB is applied in the fabrication of phenolic or epoxy resins and polycarbonates and its annual production or imports in the European Union was approximately <1 t year-1 (Serra et al. 2019; Li et al. 2022a; Owczarek et al. 2022). In turn, BPF is used as a monomer or additive in the production of epoxy resins and polycarbonate plastics applied in the fabrication of food containers, safety devices, lacquers, varnish, dental sealants and water pipes (Chen et al. 2016; Tisler et al. 2016; Liu et al. 2021; Han et al. 2022). It is assessed that the annual manufacturing /import volume of this alternative was in the range of 1000-10000 t in the European Economic Area (Wang et al. 2017a; Liu et al. 2021). Unfortunately, several studies have shown that due to the similar structure of BPs to BPA, these compounds exert a similar or greater adverse impact on the endocrine system of humans and animals (Hu et al. 2019). This is a dangerous phenomenon considering that the extensive use of these compounds results in their constantly increasing discharge into various environmental components, including surface water (Table 2).
The research currently existing in the literature primarily concentrates on the occurrence of BPA and the toxicity of this xenobiotic to aquatic organisms, while the harmful impact of bisphenol analogues and its mixtures on non-target organisms, such as green algae and their environmental risks are still mostly unknown and must be thoroughly explored. Therefore, this research aimed to examine the toxic effects of BPs, such as BPB, BPC, BPE, BPF, BPFL and BPPH, as well as their mixtures on two freshwater algal species, Chlorella vulgaris and Desmodesmus armatus. Additionally, in this study, the combined toxicity of BPs to green algae was estimated using the Keplinger evaluation system and a preliminary ecological risk assessment of the tested compounds was carried out according to Commission Directive 93/67/EEC and the risk quotient method.
Material and methods
Strains and reagents
Chlorella vulgaris (BA-2) and Desmodesmus armatus (BA-6) strains were acquired from the Culture Collection of Baltic Algae (Institute of Oceanography of the University of Gdansk, Gdansk, Poland).
Bisphenol B (BPB, >98%), bisphenol C (BPC, >98%), bisphenol E (BPB, >98%) and bisphenol PH (BPPH, >98%) were purchased from TCI (Japan), bisphenol F (BPF, >95%) and bisphenol FL (BPFL, >98%) from Fluorochem (UK). All chemicals used to prepare BG-11 medium were of analytical grade. Ultrapure water was made using a Hydrolab Spring water purification system (>18.2 MΩ cm-1 at 15 °C; Hydrolab, Poland). All solutions of BPs were prepared in methanol (HPLC grade, Chemsolve, Poland). The final methanol content in test and control samples was less than 1% (v/v) and did not affect the growth of microalgae.
Organism culturing and toxicity testing
The green algae C. vulgaris and D. armatus were grown in a climatic chamber (Pol-Eko, Poland) in mineral BG-11 medium (pH 7.64 ± 0.02) (Rippka et al. 1979) using 250 mL Erlenmeyer flasks at 26/21 °C on a 14/10 h day/night cycle with a constant humidity of 30% (60 μmol photons m-2 s-1). Based on the pre-experiment, C. vulgaris and D. armatus cultures were exposed to several concentrations (5, 10, 15, 25, 50, 75 and 100 mg L-1) of individual BPs (BPB, BPC, BPE, BPF, BPFL and a BPPH) and mixture of them (MIX, 1:1:1:1:1:1:1) for 14 days. All experiments were carried out by using algal cells in the exponential phase with an initial density of 9.6 × 104 cells mL-1 for C. vulgaris and 4.1 × 103 cells mL-1 for D. armatus. The test flasks were shaken three times a day for 1 min on a rotary shaker (100 rpm) to prevent algal cells adhering to their walls. All treatments were performed in five replicates.
Determination of pH, biomass and chlorophyll a concentration
The pH in the control and test samples was measured at 0, 4, 7, 11 and 14 days. According to the existing methodology, biomass and chlorophyll a concentrations were assessed at 0, 1, 2, 3, 4, 7, 8, 10, 11 and 14 days (Czarny et al. 2019). Briefly, to measure biomass dry weight, 10 mL of samples were taken from Erlenmeyer flasks and filtered with gentle vacuum filtration. The filters were then dried at 105 °C (1 h) to a constant mass and weighted again. Finally, the biomass content was calculated based on discrepancy between the filters' initial and final dry weight. Chlorophyll a concentrations were measured using an AlgaeChek Ultra fluorometer (Modern Water, UK). The number of microalgal cells at the beginning of the toxicity test was determined using a Fuchs-Rosenthal hemocytometer.
Data analysis
All results are represented as the mean and standard deviation (n=5). The Q-Dixon test was performed to detect outliers (Dixon 1953). Significant differences between treatments and control samples were identified by one-way ANOVA (α=0.05) using OriginPro 2023b software (OriginLab Corporation, USA).
The percentage of inhibition (PI) of BPs for C. vulgaris and D. armatus was calculated based on the chlorophyll a content by using the following equation:
where: chlc is chlorophyll a content in control sample, chlt is chlorophyll a content in test sample.
The half-concentration effect (14 day EC50) of individual BPs and their mixture on C. vulgaris and D. armatus was estimated by Microsoft Excel (Microsoft, USA) using a linear log method based on the biomass content.
The combined toxic effects of BPs was estimated using the Keplinger evaluation system (Keplinger and Deichmann 1967), based on the following formula:
where EC50,pred is predicted EC50 value and EC50,exp is experimentally obtained EC50 value.
To evaluate the potential risk of BPs to green algae, the EC50 values for these pollutants were compared to categories of concern for aquatic species defined by the European Commission Directive No. 93/67/EEC, then the compounds were classified as follows: very toxic (<1 mg L-1), toxic (1-10 mg L-1), harmful (10-100 mg L-1) and not classified (>100 mg L-1).
In addition, the ecotoxicological risk assessment of BPs was also predicted by risk quotients (RQs), using the following formula:
where MEC is measured environmental concentration and PNEC is predicted no effect concentration (EC50 value divided by an assessment factor of 1000) (Czarny-Krzyminska et al. 2023).
Based on the calculated RQ values the ecotoxicological risk of BPs can be classified into different categories, such as negligible (<0.01), low (0.01-0.1), moderate (0.1-1) and high (>1).
Results
In general biomass and chlorophyll a concentrations significantly decreased as a function of bisphenol analogue concentration and exposure time, suggesting that tested compounds had an inhibitory effect on the growth of green algae (Supplementary Tables S1-S4). The percentage of inhibition calculated based on chlorophyll a content achieved for C. vulgaris and D. armatus according to the investigated compounds are shown in Figs. 1 and 2. The inhibition effect on both green algae species increased gradually with the highest percentage of inhibition reaching up to 100% from the 7th or 8th day of incubation in the occurrence of 50-100 mg L-1 BPB, BPC for C. vulgaris and BPB, BPC, BPFL for D. armatus, respectively. For the remaining single tested compounds for which no lethal effect was observed (BPE, BPF, BPFL, PHPH for C. vulgaris and BPE, BPF, PHPH for D. armatus, respectively), the maximum percentage of inhibition was 57.11-68.79% after 14 days of exposure. Mixtures of bisphenol analogs also had harmful effects on both green algae species. As their concentrations increased (50-100 mg L-1), the maximum value of the percentage of inhibition reached 100% during the 7th day of incubation resulting in a lethal effect. However, the inhibition percentage of BPB, BPC and BPE at concentrations of 5-25 mg L-1 was up to -43.95% and stimulated algal reproduction, while the high dose (50-100 mg L-1) inhibited their growth (up to 100%). This indicates that lower concentrations of these pollutants stimulated algal cell divisions and finally showed a growth-promoting effect.
The obtained pH values are summarized in Figs. 3 and 4 for C. vulgaris and D. armatus, respectively. In control samples, the pH increases during 14-day test periods due to the consumption of dissolved CO2 (7.64-9.48 and 7.64-9.63 for C. vulgaris and D. armatus, respectively). Nevertheless, pH was decreased by single bisphenol analogues and their mixtures in a dose-dependent manner. For example, during 14 days of exposure to 5-100 mg L-1 of the tested compounds, pH changed from an initial value of 7.64 to a final value of 7.59-9.64 and 7.62-9.86 for C. vulgaris and D. armatus, respectively.
The effective concentrations (EC50) were calculated based on biomass content for individual BPs and their mixture for the 14th day of exposure of both green algae species (Table 3). The results showed that the EC50 (14 day) of BPB, BPC, BPPH and BPB, BPC, BPFL, BPPH was 33.32-43.32 and 30.49-64.54 mg L-1, which strongly affected the growth of C. vulgaris and D. armatus respectively. In turn, the impact of the remaining individual tested compounds (BPE, BPF, BPFL and BPE, BPF for C. vulgaris and D. armatus respectively) was less harmful to these green algae species (EC50 (14 day): 106.75-322.35 mg L-1). The results also indicate a strong toxic effect of mixtures of bisphenol analogs against the tested microorganisms (EC50 (14 day): 24.55-32.68 mg L-1). In addition, analyzing the obtained EC50 values, it can be seen that D. armatus (mean EC50 (14 day): 124.12 mg L-1) was a more sensitive green algal species to the tested compounds than C. vulgaris (mean EC50 (14 day): 135.49 mg L-1). Based on EC50 values, the overall toxicity ranking is MIX>BPPH>BPB>BPC>BPFL>BPF>BPE for C. vulgaris and BPB>MIX>BPFL>BPC>BPPH>BPE>BPF for D. armatus.
Discussion
As far as the authors are aware, the current investigation is the first report of toxicity of individual BPs, such as BPB, BPC, BPE, BPF, BPFL, BPPH, as well as their mixture to the microalgae C. vulgaris and D. armatus. Unfortunately, the data currently found in the literature focus only on the toxicity of BPA to green algae (Czarny-Krzyminska et al. 2023). For example Yang et al. (2021) reported that the IC50 (3 day) value of BPA to Tetraselmis sp. was as low as 3.25 mg L-1 (Yang et al. 2021). Similar results were documented by Falcao et al. (2019) with an IC50 (7 day) of BPA for Tetraselmis chuii of 0.57 mg L-1. In contrast, C. vulgaris and Chlorella pyrenoidosa were much less susceptible to BPA, the determined EC50 values for these green algae species were 39.80-63.53 mg L-1 (Ji et al. 2014; Zhang et al. 2014; Li et al. 2017; Ding et al. 2020; Czarny-Krzyminska et al. 2022). Similarly, the EC50 values for Scenedesmus obliquus and Scenedesmus quadricauda were 13.23-33.90 mg L-1 (Zhang et al. 2014; Li et al. 2017; Xiang et al. 2018). Far less studies have been dedicated to bisphenol analogues. Yadav et al. (2023) show that the EC50 (4 day) values of BPAF, BPB, BPF, BPS and BPZ to Chlamydomonas mexicana were 1.79, 12.10, 30.53, 85.49 and 9.54 mg L-1, respectively (Yadav et al. 2023). Tisler et al. (2016) documented that BPF (IC50 (3 day): 20.3 mg L-1) was less toxic than BPA (IC50 (3 day): 14.0 mg L-1) to the freshwater alga Desmodesmus subspicatus (Tisler et al. 2016). A similar trend was observed by Elersek et al. (2021), in their studies observing that BPF (EC50 (3 day): 9.2 mg L-1) was less toxic than BPA (EC50 (3 day): 6.8 mg L-1) to Pseudokirchneriella subcapitata (Elersek et al. 2021). Comparing these results with those obtained in this work, it can be concluded that C. mexicana, P. subcapitata and D. subspicatus are more sensitive to BPB and BPF than C. vulgaris and D. armatus (EC50 (14 day): 30.49-38.60 mg L-1 for BPB and 268.60-306.29 mg L-1 for BPF, respectively). Unfortunately, information about the impact of bisphenol analogies on green algae is limited and there is a lack of experiments on the harmful effects of compounds such as BPC, BPE, BPFL and BPPH.
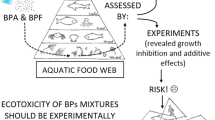
Many different bisphenol analogs can co-exist in aquatic ecosystems. Therefore, studying the interactions (synergistic, additive, or antagonistic effects) between these pollutants is very important since determining the toxic effects of only single substances may underestimate the actual risk to the ecosystems (Czarny-Krzyminska et al. 2023; Li et al. 2023). Unfortunately, even though green algae are simultaneously exposed to complex combinations of many different bisphenol analogs, only a few research have studied the harmful effects of their mixtures on these microorganisms. In this work, for the first time, the harmful effects of a mixture consisting of six emerging pollutants, such as BPB, BPC, BPE, BPF, BPFL, BPPH were investigated towards green algae. The results obtained suggest that the mixture of bisphenol analogs (EC50 (14 day): 24.55-32.68 mg L-1) is more toxic to both green algae than the individual single compounds (EC50 (14 day): 30.49-322.35 mg L-1). To assess the interaction between bisphenol analogs in the mixture, the predicted effects were determined based on the Keplinger evaluation system by calculating the combined effect coefficient (K). The calculated combined effect coefficient was 5.5 for C. vulgaris and 3.8 for D. armatus, respectively, indicating that the BPs mixture had synergistic effects on both green algae species. Similar interactions have been reported by Czarny-Krzymińska et al. (2022) in a studying the toxic impact of individual bisphenol analogues and their mixtures on C. vulgaris and D. armatus (Czarny-Krzyminska et al. 2022). In this work, a mixture of BPA and its six analogues, such as BPAF, BPG, BPM, BPP, BPX, and BPY leads to a synergistic effect (K>1.75). (Li et al. 2022b) also noted that the combined toxicity (mixture toxicity EC50 (6 day): 4.8 mg L-1) of bisphenol A (single toxicity EC50 (6 day): 17.7 mg L-1) and bisphenol S (single toxicity EC50 (6 day): 66.1 mg L-1) showed a synergistic effect against C. pyrenoidosa. On the other hand, Elersek et al. (2021) showed that a mixture of BPA and BPF towards P. subcapitata showed an additive impact at lower exposure levels and antagonism effect at higher exposure values (Elersek et al. 2021). Regrettably, the reports mentioned are the only ones available in the literature regarding the toxic effects of mixtures of analogues on green algae. Therefore, considering the interactions that occur between the complex components of mixtures, the results of these studies are useful in further understanding the harmful impact of bisphenol analogues on green algae and are important for assessing the environmental risks associated with their occurrence in aquatic ecosystems.
As in the present study, data presented in the literature show differences in the sensitivity of different algal species to bisphenol analogues (Azizullah et al. 2022). Zhang et al. (2014) in chronic toxicity tests observed that the green alga S. obliquus (concentration-dependent toxic effect) was more sensitive to BPA than C. pyrenoidosa (no toxic effect). Ji et al. (2014) also showed that C. mexicana (EC50 (5 day): 44.8 mg L-1) was less susceptible to BPA than C. vulgaris (EC50 (5 day): 39.8 mg L-1). A similar trend was noted by Xiang et al. (2018), where S. quadricauda (EC50 (4 day): 13.2 mg L-1) was more tolerant to BPA compared to Cylindrospermopsis raciborskii (EC50 (4 day): 9.7 mg L-1). Li et al. (2017) studying the toxic effect of BPA to C. pyrenoidosa (EC50 (4 day): 44.9 mg L-1) and S. obliquus (EC50 (4 day): 33.9 mg L-1), indicated that the different responses among these two green algae species may be attributable to variations in the composition of their cell walls. Thus, it can be assumed that rhamnose and cellulose are responsible for the rigidity of the cell wall of the unicellular C. vulgaris (Safi et al. 2014), while the cell wall of D. armatus, which often occurs in the form of four- to eight-celled coenobia, consists predominantly of mannose, which is why it is more sensitive to these compounds (Takeda 1995; Dunker and Wilhelm 2018). In addition, many studies indicate that microorganisms such as green algae are capable of absorbing, accumulating, biodegrading and biotransforming bisphenol A, and its analogues (Ji et al. 2014; Wang et al. 2017b; Ding et al. 2020; He et al. 2022). Hence, different species of green algae differ in their ability to degrade these compounds, and the resulting various degradation products may differ in toxicity. This phenomenon may be another explanation for the differential sensitivity of the microalgae C. vulgaris and D. armatus to bisphenol analogues.
Bisphenol analogs are compounds newly introduced on the market, so their mechanism of action against green algae is not well understood as yet and depends on many complex factors. Based on the data presented in Table 1 (molecular structures and physicochemical properties), the behavior and fate of BPs in the environment can be partially predicted. The bisphenol analogues studied in this work are lipophilic compounds (logP: 3.06-7.17) that can easily pass through the cell walls of green algae. They can then inhibit the development of C. vulgaris and D. armatus by directly affecting the division of cells or indirectly by negatively affecting various physiological and biochemical processes (Azizullah et al. 2022). In turn, the use of the ECOSAR program allows for estimating the toxicity of new or untested substances, such as bisphenol analogues. The toxicity calculated from this program indicates that BPB, BPC, BPFL and BPPH (EC50 (4 day): 0.041-0.964 mg L-1) show higher toxicity than BPA (EC50 (4 day): 1.33 mg L-1) against green algae. In contrast, comparing the experimental data obtained in this work with literature data (Czarny-Krzyminska et al. 2022), it can be seen that BPB, BPPH (EC50 (14 day): 33.32-38.60 mg L-1) for C. vulgaris and BPB, BPFL (EC50 (14 day): 30.49-35.07 mg L-1) for D. armatus show more toxic effect than BPA (EC50 (14 day): 42.06-42.52 mg L-1). Hence, these compounds should not be used as substitutes of BPA in the manufacturing of consumer products. Harmful impacts of bisphenol analogs on green algae can be caused by several pathways, and the occurring synergistic/additive/antagonistic effects, growth-stimulating effects, biotransformation of compounds into different types of degradation products differing in toxicity makes experimental data more reliable for ecotoxicological risk assessments than those predicted by the QSAR model. Numerous studies have shown that exposure of microalgae to BPA causes changes in cell morphology. Research demonstrated that this compound leads to the breaking of the cell wall, disorganization of cell organelles and their damage, inhibition of cell division, and fading of color indicating the disintegration of chloroplasts and chlorophyll particles (Li et al. 2008, 2009; Xiang et al. 2018). Determination of chlorophyll a concentration during ecotoxicological testing is usually used to assess the photosynthetic effect of green algae (Ding et al. 2020; Li et al. 2021). In this study, a concentration-dependent reduction in chlorophyll a concentration was noticed in C. vulgaris and D. armatus exposed to bisphenol analogues, suggesting that these compounds can inhibit the synthesis of light-harvesting pigments. These observations are consistent with data available in the literature, which indicated that BPA and its analogues negatively affects light-harvesting pigments in microalgae (Li et al. 2008, 2009; 2021; Zhang et al. 2014; Xiang et al. 2018; Ding et al. 2020). Li et al (2008) showed that reduced chlorophyll content in Cyclotella caspia cells exposed to BPA is associated with chloroplast destruction. In addition, Zhang et al. (2014) documented that BPA inhibited the chlorophyll content of C. pyrenoidosa and S. obliquus after 6 days of exposure, which can be attributable to the interference of this compound with the protochlorophyll synthesis, and its conversion to chlorophyll. During photosynthesis green algae consume inorganic carbon and accumulate hydroxyl ions in the medium, which leads to an increase in its pH (de Morais and Costa 2007). Therefore, it can be concluded that the significantly lower final pH values obtained in this work for higher levels of bisphenol analogs (50-100 mg L-1) (pH: 7.59-9.35 and 7.62-9.60 for C. vulgaris and D. armatus, respectively) compared to the control sample (pH: 9.48 and 9.63 for C. vulgaris and D. armatus, respectively) indicate that increasing doses of the toxicant inhibited photosynthesis. These findings were consistent with observations presented by Ji et al. (2014) that the final pH for C. vulgaris exposed to 50 mg L-1 of BPA was almost the same as the initial values. Similarly, Wang et al. (2017b) reported that photosynthetic activity (Fv/Fm) and pH of Desmodesmus sp.WR1 was inhibited during exposure to BPA at concentrations above 3 mg L-1. In turn, Duan et al. (2019) showed that high concentrations of BPA (10 mg L-1) inhibited changes in the expression levels of several pathways that were associated with the tricarboxylic acid cycle, fatty acid metabolism, glycolysis, oxidative phosphorylation and photosynthesis. In addition, many studies indicate that BPA induces oxidative stress in many microalgae species, as evidenced by increased lipid peroxidation (malondialdehyde) and antioxidant enzyme activity (superoxide dismutase, catalase) (Li et al. 2008; Liu et al. 2010; Zhang et al. 2014; Wang et al. 2017b; Ben Ouada et al. 2018; Xiang et al. 2018). In summary, studies available in the literature, indicate that the toxic impact of BPA and its analogs may be due to multiple pathways and involve impairment of various metabolic processes taking place in green algae cells as a result, and can take the form of growth inhibition.
The adverse effects of bisphenol analogues on the environment require further study, as such compounds are only just being introduced to the market as replacements for BPA and their fate in the environment has not precisely known yet. The ecological risk assessment of single substances is extremely valuable and important. However, it does not take into account simultaneous exposure to numerous contaminants. As previously mentioned, in aquatic ecosystems organisms are exposed to complex mixtures of compounds. In the present study, the results have confirmed that bisphenol analogues are toxic to green algae, suggesting that these compounds may pose some ecological risks in the aquatic environment. Therefore, this paper estimates the potential risk to aquatic organisms associated with the occurrence of individual compounds and their mixtures in the environment based on Commission Directive No. 93/67/EEC and RQ classifications. According to the EU Commission Directive No. 93/67/EEC compounds such as BPB, BPC, BPPH, MIX (EC50 (14 day): 24.55-43.32 mg L-1) and BPB, BPC, BPFL, BPPH, MIX (EC50 (14 day): 30.49-64.54 mg L-1) can be regarded as harmful to C. vulgaris and D. armatus, respectively (Fig. 5a). However, this classification does not take into account the concentration of these compounds in the environment. Therefore, in this work, the risk quotient (RQ) method was used to quantitatively characterize the risk of chemicals. Fig. 5(b) summarizes the obtained RQ values for tested bisphenol analogues. Of the six investigated compounds and their mixtures, only BPF, MIX (for C. vulgaris) and MIX (for D. armatus) showed low ecological risk (RQ: 0.011-0.019). For the other bisphenol analogues, the RQ values were <0.01, indicating an insignificant risk to organisms. Although the concentrations of the individual compounds in the aquatic ecosystems are insignificant (ng L-1 to μg L-1) to pose a serious threat to green algae, this study indicates that their mixtures may pose a low risk to aquatic organisms. This is especially hazardous because in the aquatic ecosystems, these compounds are usually present in complex mixtures. BPA, which is a high-production volume chemical, is increasingly banned or restricted during the manufacture of many everyday products and replaced by its analogues. Therefore, the environmental levels of these pollutants in the aquatic ecosystems are underestimated and are expected to increase continuously. In addition, it should be noted that the risk assessment for bisphenol analogues is based on the few available data on its concentrations in the aquatic ecosystems. Therefore, the environmental levels of these pollutants may be underestimated and are expected to increase continuously. Accordingly, in the future, the risks associated with their occurrence in the ecosystems may be much higher and the environmental levels of these compounds should be continuously monitored.
Conclusions
Given their structural similarity to BPA and ubiquitous occurrence in environments, bisphenol analogs have gained increasing attention, especially in terms of their safety for aquatic organisms. Unfortunately, only a few researchers have studied the potential toxic effects of bisphenol analogues on green algae, which are an important group of primary producers. Therefore, in this study, the toxic effects and potential ecotoxicological risks of bisphenol analogues occurring in the aquatic ecosystems (BPB, BPC, BPE, BPF, BPFL, BPPH) on two freshwater algae C. vulgaris and D. armatus were examined. The findings showed that BPB, BPC, BPPH (EC50 (14 day): 33.32-43.32 mg L-1) and BPB, BPC, BPFL, BPPH (EC50 (14 day): 30.49-64.54 mg L-1) were most toxic to cells of C. vulgaris and D. armatus, respectively. In turn, the remaining bisphenol analogues (BPE, BPF, BPFL for C. vulgaris and BPE, BPF for D. armatus, respectively) were less toxic to these green algae species. Moreover, the toxicity of the mixture of tested bisphenol analogues was higher (EC50 (14 day): 106.75-322.35 mg L-1) than the sum of the toxicity of its individual componentsshowing that co-exposure to bisphenol analogues leads to synergistic effects (combined effect coefficient: 3.8-5.5). These findings are extremely important, because in aquatic ecosystems microalgae are exposed to complex mixtures of pollutants, and the occurrence of synergistic interactions caused that ecotoxicological risk assessment only on the basis of the toxicity of single compounds may be underestimated. The toxic rankings of individual bisphenol analogues and their mixture to C. vulgaris and D. armatus waswere MIX>BPPH>BPB>BPC>BPFL>BPF>BPE and BPB>MIX>BPFL>BPC>BPPH>BPE>BPF, respectively. In this research, the ecotoxicological risk of bisphenol analogues has been predicted based on Commission Directive No. 93/67/EEC and the risk quotient (RQ) method. In compliance with Commission Directive No. 93/67/EEC compounds such as BPB, BPC, BPPH, MIX (EC50 (14 day): 24.55-43.32 mg L-1) and BPB, BPC, BPFL, BPPH, MIX (EC50 (14 day): 30.49-64.54 mg L-1) can be considered as harmful to the green algae C. vulgaris and D. armatus, respectively. Nevertheless, this classification does not take into account the environmental levels of these compounds. Therefore, the risk quotient method was also used to estimate ecotoxicological risks. The calculated RQs for BPF, MIX (for C. vulgaris) and MIX (for D. armatus) were 0.011-0.019, indicating low ecological risk. For the remaining bisphenol analogs, RQ values were <0.01, suggesting insignificant risk to both green algae species. Nevertheless, it is likely that this value will escalate to more harmful levels due to the increasing usage of these compounds as BPA substitutes. To summarize, the toxicity of BPA to green algae has been fairly well studied, but information on the potential effects of many bisphenol analogs is lacking. Therefore, further investigation on the toxicity of these pollutants and their levels in the environment are needed to fully assess the ecotoxicological risk to aquatic organisms, such as green algae.
Data availability
The datasets used in the current study are available from the corresponding author upon reasonable request.
References
Abraham A, Chakraborty P (2020) A review on sources and health impacts of bisphenol A. Rev Environ Health 35:201–210
Azizullah A, Gao KS, Khan S, Gao G (2022) The interplay between bisphenol A and algae-A review. J King Saud Univ Sci 34:102050
Ben Ouada S, Ben Ali R, Leboulanger C, Ben Ouada H, Sayadi S (2018) Effect of Bisphenol A on the extremophilic microalgal strain Picocystis sp. (Chlorophyta) and its high BPA removal ability. Ecotoxicol Environ Saf 158:1–8
Chen D, Kannan K, Tan H, Zheng Z, Feng YL, Wu Y, Widelka M (2016) Bisphenol analogues other than BPA: Environmental occurrence, human exposure, and toxicity-A review. Environ Sci Technol 50:5438–5453
Czarny-Krzyminska K, Krawczyk B, Szczukocki D (2022) Toxicity of bisphenol A and its structural congeners to microalgae Chlorella vulgaris and Desmodesmus armatus. J Appl Phycol 34:1397–1410
Czarny-Krzyminska K, Krawczyk B, Szczukocki D (2023) Bisphenol A and its substitutes in the aquatic environment: Occurrence and toxicity assessment. Chemosphere 315:137763
Czarny K, Szczukocki D, Krawczyk B, Skrzypek S, Zielinski M, Gadzala-Kopciuch R (2019) Toxic effects of single animal hormones and their mixtures on the growth of Chlorella vulgaris and Scenedesmus armatus. Chemosphere 224:93–102
de Morais MG, Costa JA (2007) Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. J Biotechnol 129:439–445
Ding T, Li W, Yang M, Yang B, Li J (2020) Toxicity and biotransformation of bisphenol S in freshwater green alga Chlorella vulgaris. Sci Total Environ 747:141144
Dixon W (1953) Processing data for outliers. Biometrics 9:74–89
Duan L, Chen Q, Duan S (2019) Transcriptional analysis of Chlorella pyrenoidosa exposed to Bisphenol A. Int J Environ Res Public Health 16:1374
Dunker S, Wilhelm C (2018) Cell wall structure of coccoid green algae as an important trade-off between biotic interference mechanisms and multidimensional cell growth. Front Microbiol 9:719
Durovcova I, Kyzek S, Fabova J, Makukova J, Galova E, Sevcovicova A (2022) Genotoxic potential of bisphenol A: A review. Environ Pollut 306:119346
ECHA (2017) Agreement of the Member State Committee on the identification of 4,4’-isopropylidenediphenol (bisphenol A) as a Substance of Very High Concern. European Chemicals Agency (ECHA). https://echa.europa.eu/documents/10162/6e9412a4-1a2c-d654-efc0-700e3e46369a
Elersek T, Notersberg T, Kovacic A, Heath E, Filipic M (2021) The effects of bisphenol A, F and their mixture on algal and cyanobacterial growth: from additivity to antagonism. Environ Sci Pollut Res Int 28:3445–3454
Falcao VGO, Carneiro DC, Pereira SA, da Silva MRD, Cande AA, da Cunha Lima ST (2019) Analyzing the toxicity of bisphenol-A to microalgae for ecotoxicological applications. Environ Monit Assess 192:8
Hamed EM, Li SFY (2022) Molecularly imprinted polymers-based sensors for bisphenol-A: recent developments and applications in environmental, food and biomedical analysis. Trends Environ Anal Chem 35:e00167
Han Y, Liu Y, Wang M, Xue Y (2022) Effects of BPZ, BPC, BPF, and BPS exposure on adult zebrafish (Danio rerio): accumulation, oxidative stress, and gene expression. Int J Environ Res Public Health 19:15784
He DD, Zeng YM, Zhou GM (2022) The influence of microplastics on the toxic effects and biodegradation of bisphenol A in the microalgae Chlorella pyrenoidosa. Aquat Ecol 56:1287–1296
Hu Y, Zhu Q, Yan X, Liao C, Jiang G (2019) Occurrence, fate and risk assessment of BPA and its substituents in wastewater treatment plant: A review. Environ Res 178:108732
Ji M-K, Kabra A, Choi J, Hwang J-H, Kim J, Abou-Shanab R, Oh Y-K, Jeon B-H (2014) Biodegradation of bisphenol A by the freshwater microalgae Chlamydomonas mexicana and Chlorella vulgaris. Ecol Eng 73:260–269
Keplinger ML, Deichmann WB (1967) Acute toxicity of combinations of pesticides. Toxicol Appl Pharmacol 10:586–595
Lehmler HJ, Liu B, Gadogbe M, Bao W (2018) Exposure to Bisphenol A, Bisphenol F, and Bisphenol S in U.S. adults and children: The National Health and Nutrition Examination Survey 2013–2014. ACS Omega 3:6523–6532
Li D, Bi R, Chen H, Mu L, Zhang L, Chen Q, Xie H, Luo Y, Xie L (2017) The acute toxicity of bisphenol A and lignin-derived bisphenol in algae, daphnids, and Japanese medaka. Environ Sci Pollut Res Int 24:23872–23879
Li HM, Li YY, Zhang YC, Li JB, Xu HM, Xiong YM, Qin ZF (2022a) Bisphenol B disrupts testis differentiation partly via the estrogen receptor-mediated pathway and subsequently causes testicular dysgenesis in Xenopus laevis. Ecotoxicol Environ Saf 236:113453
Li J, Wang Y, Li N, He Y, Xiao H, Fang D, Chen C (2022b) Toxic effects of Bisphenol A and Bisphenol S on Chlorella pyrenoidosa under single and combined action. Int J Environ Res Public Health 19:4245
Li J, Li W, Huang X, Ding T (2021) Comparative study on the toxicity and removal of bisphenol S in two typical freshwater algae. Environ Sci Pollut Res Int 28:36861–36869
Li R, Chen GZ, Tam NF, Luan TG, Shin PK, Cheung SG, Liu Y (2009) Toxicity of bisphenol A and its bioaccumulation and removal by a marine microalga Stephanodiscus hantzschii. Ecotoxicol Environ Saf 72:321–328
Li R, Liu Y, Chen G, Tam N, Shin P, Cheung S, Luan T (2008) Physiological responses of the alga Cyclotella caspia to bisphenol A exposure. Bot Mar 51:360-369
Li W, Lv L, Wang Y, Zhu YC (2023) Mixture effects of thiamethoxam and seven pesticides with different modes of action on honey bees (Aplis mellifera). Sci Rep 13:2679
Liu J, Zhang L, Lu G, Jiang R, Yan Z, Li Y (2021) Occurrence, toxicity and ecological risk of Bisphenol A analogues in aquatic environment - A review. Ecotoxicol Environ Saf 208:111481
Liu Y, Guan Y, Gao Q, Tam NF, Zhu W (2010) Cellular responses, biodegradation and bioaccumulation of endocrine disrupting chemicals in marine diatom Navicula incerta. Chemosphere 80:592–599
Mukhopadhyat R, Prabhu N, Kabekkodu S, Rai P (2022) Review on bisphenol A and the risk of polycystic ovarian syndrome: an insight from endocrine and gene expression. Env Sci Pollut Res 29:32631–32650
Owczarek K, Waraksa E, Kłodzińska E, Zrobok Y, Ozimek M, Rachoń D, Kudłak B, Wasik A, Mazerska Z (2022) Validated GC–MS method for determination of bisphenol a and its five analogues in dietary and nutritional supplements. Microcheml J 180:107643
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Safi C, Zebib B, Merah O, Pontalier P, Vaca-Gracia C (2014) Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew Sustain Energy Rev 35:265–278
Serra H, Beausoleil C, Habert R, Minier C, Picard-Hagen N, Michel C (2019) Evidence for Bisphenol B endocrine properties: Scientific and regulatory perspectives. Environ Health Perspect 127:106001
Sirasanagandla SR, Al-Huseini I, Sakr H, Moqadass M, Das S, Juliana N, Abu IF (2022) Natural products in mitigation of bisphenol A Toxicity: future therapeutic use. Molecules 27:5384
Takeda H (1995) Cell wall sugars of some Scenedesmus species. Phytochemistry 42:673–675
Tisler T, Krel A, Gerzelj U, Erjavec B, Dolenc MS, Pintar A (2016) Hazard identification and risk characterization of bisphenols A, F and AF to aquatic organisms. Environ Pollut 212:472–479
Wang Q, Chen M, Shan G, Chen P, Cui S, Yi S, Zhu L (2017) Bioaccumulation and biomagnification of emerging bisphenol analogues in aquatic organisms from Taihu Lake, China. Sci Total Environ 598:814–820
Wang R, Diao P, Chen Q, Wu H, CXu N, Duan W, (2017) Identification of novel pathways for biodegradation of bisphenol A by the green alga Desmodesmus sp.WR1, combined with mechanistic analysis at the transcriptome level. Chemical Engineering Journal 321:424–431
Xiang R, Shi J, Yu Y, Zhang H, Dong C, Yang Y, Wu Z (2018) The effect of Bisphenol A on growth, morphology, lipid peroxidation, antioxidant enzyme activity, and PS II in Cylindrospermopsis raciborskii and Scenedesmus quadricauda. Arch Environ Contam Toxicol 74:515–526
Xing J, Zhang S, Zhang M, Hou J (2022) A critical review of presence, removal and potential impacts of endocrine disruptors bisphenol A. Comp Biochem Physiol C 254:109275
Yadav N, Ahn HJ, Kurade MB, Ahn Y, Park YK, Khan MA, Salama ES, Li X, Jeon BH (2023) Fate of five bisphenol derivatives in Chlamydomonas mexicana: Toxicity, removal, biotransformation and microalgal metabolism. J Hazard Mater 454:131504
Yang Q, Xu W, Luan T, Pan T, Yang L, Lin L (2021) Comparative responses of cell growth and related extracellular polymeric substances in Tetraselmis sp. to nonylphenol, bisphenol A and 17α-ethinylestradiol. Environ Pollut 274:116605
Zhang W, Xiong B, Sun WF, An S, Lin KF, Guo MJ, Cui XH (2014) Acute and chronic toxic effects of bisphenol A on Chlorella pyrenoidosa and Scenedesmus obliquus. Environ Toxicol 29:714–722
Funding
This work was financially supported by the National Science Center (NCN) in Krakow, Poland (Grant no. 2020/04/X/NZ8/01436).
Author information
Authors and Affiliations
Contributions
Karolina Czarny-Krzymińska: Conceptualization, Methodology, Formal analysis, Investigation, Funding acquisition, Writing - Original Draft, Writing- Review & Editing, Visualization. Barbara Krawczyk: Supervisions. Dominik Szczukocki: Supervisions.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Credit author
Karolina Czarny-Krzymińska: Conceptualization, Methodology, Formal analysis, Investigation, Funding acquisition, Writing - Original Draft, Writing- Review & Editing, Visualization. Barbara Krawczyk: Supervisions. Dominik Szczukocki: Supervisions.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Czarny-Krzymińska, K., Krawczyk, B. & Szczukocki, D. Toxic effects of bisphenol analogues and their mixture on two freshwater algae Chlorella vulgaris and Desmodesmus armatus. J Appl Phycol (2024). https://doi.org/10.1007/s10811-024-03289-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10811-024-03289-9