Abstract
Nano-filtered whey permeate (WP), a major by-product of dairy industry, is produced by membrane filtration of whey. The oleaginous microalga Nannochloropsis oceanica was successfully cultivated on WP without salinity and nutrient amendments. Growth, cell characteristics, and fatty acid profile of the cultures were analyzed using microscopy, flow cytometry, and GC analysis. WP was nitrogen limited, comprising primarily protein as a nitrogen source and only small amounts of free inorganic nitrogen (in the form of nitrate). Nannochloropsis oceanica (and associated bacteria) efficiently removed nitrate (100%), protein (87%), and phosphate (74%) from the whey permeate. Microscopic and flow cytometric analysis revealed diverse size distributions in whey permeate cultures, with significant cell aggregation attributed to low-salinity acclimatization and nitrogen limitation. Autofluorescence analysis revealed reduced photosynthetic activity in whey permeate-grown cells, possibly as a consequence of heightened mixotrophic activities on carbon source in the medium. Low nitrogen availability in whey permeate resulted in biomass with a fatty acid profile enriched in saturated fatty acids. Despite this, a considerable level of the omega-3 polyunsaturated fatty acid (in the form of eicosapentanoic acid or EPA) was detected at ca. 16% of total fatty acids. Whey permeate proved beneficial for the growth of N. oceanica and yielded high concentrations of eicosapentaenoic acid in the extracted lipids for potential applications in the feed/food industries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The dairy industry is a critical food sector in Europe and worldwide. The industry generates large amounts of wastewater and byproducts with high levels of nitrogen and phosphate, which can be challenging to treat and thus accrue a significant cost burden in treatment process. However, these nutrients can potentially support the growth of microalgae. Casein whey permeate, a food-grade byproduct of dairy industry, has the potential to be used for sustainable omega-3 polyunsaturated fatty acids (ω-3 PUFAs) production by microalgae since it contains nutrients and minerals suitable for microalgae growth such as phosphate and potassium (Kiani et al. 2022). Using byproduct as a microalgal growth medium offers a win-win synergy that can reduce the costs to produce these valuable compounds for the food and feed/aquaculture industries, while at the same time offering the dairy industry the means to valorise their waste in a sustainable approach.
The raw whey generated from cheese and casein production can be processed using nano-filtration, generating a retentate stream rich in lactose and protein for potential application in animal feed. The residual product of nano-filtration process is a permeate that is rich in minerals and salts and contains small amounts of lactose and protein. The nano-filtered whey permeate (referred to as WP throughout the study) is a byproduct that is difficult to treat with conventional wastewater treatment methods due to its high salinity, high phosphate content, and low organic carbon content. Microalgae have previously been demonstrated to grow in media with high salt contents and can thus serve as a robust solution for the treatment of WP (Qie et al. 2019; Shahid et al. 2020; Kiani et al. 2022).
Several investigations have employed microalgae to treat wastewater from cheese and casein powder manufacture. Daneshvar et al. (2018) analysed freshwater (Scenedesmus quadricauda) and marine (Tetraselmis suecica) microalgae to bioremediate dairy wastewater and confirmed that both microalgae can treat the waste to produce lipids. Chokshi et al. (2016) showed that Acutodesmus dimorphus can achieve complete ammonium removal from dairy wastewater. Pandey et al. (2020) investigated a two-step process (coagulation and microalgae cultivation) to simultaneously treat the dairy wastewater and produce lipids for biofuel application. Lucakova et al. (2022) cultured Spirulina sp. on whey from cheese manufacture and demonstrated that a growth system based on waste recovery was able to diminish the cost of biomass production significantly. Ghobrini et al. (2020) studied the cultivation of Chlorella vulgaris on dairy wastewater with high salt concentration and showed that microalgae can be used for the treatment of wastewater. de Almeida Pires et al. (2021) used C. vulgaris for the treatment of cheese-whey wastewater and showed that waste treatment effectiveness was boosted at high initial cell concentrations. None of these studies, however, has investigated the potential of dairy waste for growing Nannochloropsis, a genus of microalgae rich in ω-3 PUFAs in the form of eicosapentanoic acid (EPA), and the impact of such growth on biomass EPA production. Furthermore, to the best of our knowledge, no studies to date have used nano-filtered WP as growth medium for microalgae.
Oleaginous microalgae such as Nannochloropsis, Tetraselmis, Phaeodactylum, Schizochytrium, Thraustochytrium, etc., are widely known for their capability to produce ω-3 PUFAs (Adarme-Vega et al. 2014; Chen et al. 2018). Nannochloropsis overproduces EPA with favourable large-scale cultivation potential, such as contamination resistance (Chen et al. 2018; Halim et al. 2019). The EPA content in Nannochloropsis cells can range between around 1-13% of the biomass depending on the cultivation conditions and species used (Xiao et al. 2013; Lai 2015; Xiao et al. 2015; Zhang et al. 2021), allowing them to be potentially employed as a replacement for fish oil in feed and food applications (Wan et al. 2013; Lee and Han 2015). Nutrient availability and balance during cultivation can considerably influence microalgal growth and lipid accumulation. As a key element of nucleic acids and structural protein, nitrogen is critical for cell division. The concentration and type of nitrogen source in growth medium therefore have a substantial effect on the biomass growth and subsequent lipid accumulation. In general, nitrogen (N), phosphorus (P) and N:P ratio played an important interactive role in the biomass productivity, although many other factors such as pH, salinity, other nutrient content, etc., could also affect the results (Chokshi et al. 2016; Chen et al. 2018; Choi et al. 2018; Chandra et al. 2021; Kiani et al. 2022).
Many reports indicated that nitrogen deficient medium may enhance microalgal-based lipid production (Pal et al. 2011; Roleda et al. 2013). Based on the literature (Huo et al. 2020) N:P ratios less than 5:1 could cause nitrogen limitation in the cells, while N:P ratios larger than 30:1 could result in phosphorus limitation. According to Makut et al. (2019), P has a complex and interactive role in the biomass synthesis. They used a response surface model to show that low phosphate concentration does not necessarily correlate with high lipid content. On the other hand, N limitation has consistently been shown to promote lipid accumulation in the microalgae (Simionato et al. 2013; Popko et al. 2016).
Omega-3 polyunsaturated fatty acids (ω-3 PUFAs) offer numerous health benefits, including cardio-protection, lipid lowering, anti-inflammatory, anti-cancer (especially breast, prostate, colon, and renal cancers), anti-Alzheimer's disease, and anti-depression effects (Singh et al. 2012; Wani et al. 2015; Zárate et al. 2017; Verveniotis et al. 2018; Bendimerad-Benmokhtar et al. 2019). They also serve as precursors for various regulatory eicosanoids. The global demand for these compounds has risen significantly, due to their widespread use as nutrient supplements in feed and food applications (Oliver et al. 2020; Magoni et al. 2022). However, commercial production of ω-3 PUFAs presently relies on fish oil derived from wild-caught fish, placing further strain on an already limited oceanic fish stock. To address sustainability concerns, alternative sources of ω-3 PUFAs are urgently needed.
In this study we investigated the development of microalgae technology based on Nannochloropsis oceanica as a potentially sustainable and cost-effective process for the simultaneous production of omega-3 oil for food/feed application and treatment of nano-filtered WP from acid casein production. Fluorescent microscopy and flow cytometry were extensively used to study population dynamics and lipid production.
Materials and methods
Nano-filtered whey permeate samples and chemical analysis
The term WP in this study refers to nano-filtered whey permeate. WP was obtained from Arrabawn Co op’s dairy manufacturing site in Nenagh (Ireland). The permeate was produced by acidic separation of casein followed by nanofiltration to remove proteins and lactose. WP samples were collected in clean containers and immediately stored at -20 °C until use. Upon thawing, the samples were autoclaved at 121 °C for 15 min to remove any bacterial contamination and then pH adjusted to 7.75 by 1 N NaOH. No further treatment such as filtration or centrifugation was performed.
Chemical analysis of WP samples was conducted prior to microalgal inoculation using standard colorimetric methods for protein, nitrate, ammonium, and phosphate determination. Protein was measured by the Bradford method (Kruger 2009) and the reagent was from Sigma-Aldrich (Germany). Nitrate and ammonium were analyzed using API standard kits (Mars Fishcare, USA). Phosphate was analyzed using standard kits from Red Sea (Seahorse, Ireland). The absorbance for each nutrient was recorded at the recommended wavelength of the respective chemical by a spectrophotometer (SpectraMax M3, Molecular Devices, UK): nitrate at 545 nm, ammonium at 690 nm, phosphate at 675 nm. Each nutrient was quantified by comparing the absorbance values to a linear calibration curve of standard solutions. Salinity was measured by a conductivity meter (RS PRO Conductivity Meter, Radionics, Ireland). To estimate the total nitrogen content (N, mg L-1), conversion factors of 0.226, 0.820, and 0.150 were used, respectively, to convert nitrate, ammonium, and protein concentration to N concentration. To convert phosphate and protein content to total P, conversion factors of 0.326 and 0.00224 were used. The conversion factors for nitrate, ammonium, and phosphate were calculated based on the molecular weight of compounds and species. For protein, standard conversion factors found in literature were used (Moore and Stanitski 2014; Bryson and Willis 2017). Table 1 shows the nutrient composition of WP used in this study.
Inoculum preparation
Nannochloropsis oceanica (CCAP 849/10) was obtained from Culture Collection of Algae and Protozoa, Scotland (CCAP, https://www.ccap.ac.uk/, Oban, Scotland, UK). Based on the information provided by the supplier, the culture was not axenic and contained associated bacterial consortia. The culture was not subjected to purification prior to use in the investigation. Cultures used in inoculum preparation and growth experiments were thus non-axenic. Inoculum cultures were cultivated in standard medium f/2 (CCAP recipe) in cell culture flasks (75 mL, working volume = 25 mL) with filtered caps. The flasks were shaken at 100 rpm at 25 °C under fluorescent light on a 12 h:12 h light:dark cycle with the intensity of 100 µmol photons m-2 s-1. The inoculum cultures were sub-cultured at exponential growth phase for inoculation in growth experiments using nano-filtered WP. The inoculum volume was 2.5 mL for each experiment.
Growth experiments
Microalgae growth experiments were performed in flat 75 mL cell culture flasks (working volume = 25 mL) at 25°C under fluorescent light with 12/12 h on/off cycle and constant agitation for 14 days using an orbital incubator shaker (Innova 44, New Brunswick, USA). The flasks were equipped with permeable caps to provide gas exchange during agitation. Microalgae inoculum was added to undiluted nano-filtered WP. The pH of the medium was adjusted to 7.8 by the addition of NaOH. No other nutrients or salts apart from those already available in the nano-filtered WP medium were added to the cultures. Sterile practice was adopted with all culture preparation steps taking place inside a safety cabinet. Control experiments were carried out by growing cultures on standard f/2 medium to compare the results.
The optical density of the culture (OD750) was measured and OD750 was converted to biomass concentration based on Eqs. 1 and 2 for the cultures grown on standard medium and WP, respectively. To develop the equations, cultures of known OD750 values in the respective medium at exponential growth stage were previously freeze dried and resultant biomass gravimetrically measured to establish correlations.
where X is the biomass concentration (g L-1).
Light and fluorescence microscopy
For microscopic cell analysis culture samples were stained with Trypan Blue, transferred onto a haemocytometer, and images captured by an upright optical microscope (CK X41, Olympus, Ireland). Images of the cells were then analysed by ImageJ (version 1.53k) software to obtain cell density and size.
A Zeiss Axio Imager M1 microscope (Carl Zeiss, Germany) equipped with an AxioCam MRs digital camera was used for all fluorescent analyses. Microalgae culture samples were stained with BODIPY (Invitrogen, Thermo Fisher, Ireland) dissolved in DMSO (1 mg mL−1) to observe lipid bodies. Imaging was conducted using filter set 38 with excitation at 470/40 nm and bandpass emission at 525/50 nm. Calcofluor white (CfW) stain (Sigma-Aldrich) (1 mM in water) was employed to observe structural polysaccharides. To this end, culture samples were stained with CfW stain (100 μM stock solution) and the fluorescent signal was observed using filter set 49 with excitation at 365 nm and bandpass emission at 445/50 nm. All stain solutions were added to the cell suspension in a ratio of 1:10 (v/v).
Flow cytometry
A Beckman Coulter CytoFlex flow cytometer (Beckman Coulter, USA) was used to analyse microalgae cells. Culture samples were collected every week from growth experiments. The algal cells were analysed initially based on their autofluorescence. The samples were excited by 350 nm laser and emission measured at 450/40 nm. Cell population with autofluorescence at this region was gated and identified as microalgae cells. Algal cells were then counted by the cytometer and clustered in three groups of small, medium, and large cells based on their forward-scatter intensity (FSC). The gated cells were also excited by a blue laser (B) (488 nm) and emission measured at 690/50 nm to indicate chlorophyll content of the cells. This allows the cells to also be clustered according to their chlorophyll contents as low, medium, and high chlorophyll containing cells.
Flow cytometry analysis was also carried out on culture samples that have been stained for lipids by BODIPY (excited by blue laser at 488 nm and measured at 525/40 nm) and structural polysaccharides by CfW (excited by NUV laser at 350 nm and measured at 450/40 nm).
Fatty acid analysis
Total lipid content of each biomass was determined using a modified version of Bligh and Dyer extraction based on chloroform/methanol/water (1:2:0.8 v/v/v) solvent extraction mixture (Halim et al. 2019). Chloroform phases containing the extracted lipid were collected from all extraction steps, pooled, filtered (0.2 μm syringe filter), and dried under flowing N2 gas. The extracted lipid was dissolved again in chloroform/methanol solution (2:1 v/v) and stored at -20 °C. To esterify the extracted lipid into fatty acid methyl esters (FAME), the lipid solution was dried, redissolved in 2 % H2SO4 in methanol, and incubated at 50 °C for 2 h. Saturated salt solution and hexane were added (ratio of 1:5) to the lipid solution to achieve biphasic separation and partitioning of FAMEs in the organic phase. After vortexing the top hexane layer containing FAMEs was transferred to a new tube. Hexane addition step was repeated twice to further extract FAMEs. Finally, all hexane phases were pooled. evaporated under N2 gas, and the FAMEs were resuspended in a known amount of hexane for chromatographic analysis.
FAME solution was analysed with a gas chromatograph equipped with a flame ionisation detector (Agilent Technologies, Ireland) to evaluate fatty acid profile. A HP-5 capillary column (Agilent Technologies) with a length of 30 m was employed and helium was used as the carrier gas at a split ratio of 10:1 and a flow rate of 2 mL min-1.
The FAMEs were separated based on their boiling point and a four-step temperature profile was used including an initial temperature at 50 °C for 2 min, increase to 150 ℃ at 10 ℃ min-1 for 2 min, increase to 185 ℃ at 10 ℃ min-1 for 2 min, and a final increase to 300 ℃ at 10 ℃ min-1 for 2 min. The detector temperature was set at 320 °C and the injection temperature was 280 ℃. Individual FAMEs were identified by retention-time comparison with a 37-component FAME standard mix (Sigma Aldrich, Ireland).
Data analysis and statistical evaluation
Growth experiments in this study were carried out in duplicates. Results are reported as mean ± standard deviation (SD). Minitab software (Version 21, Minitab LLC, USA) was used to carry out analysis of variance (one way ANOVA) and means comparison tests. Flow cytometry population data were analysed by CytExpert software (Varsion 2.5, Beckman Coulter, USA).
Results
Nutrient removal
Figure 1 illustrates the percentage of nutrient removal from WP attained by N. oceanica at the end of cultivation. The highest reduction was observed for nitrate, followed by protein and phosphate. Nitrate was entirely consumed by Nannochloropsis cells showing that this source of N was preferred by the microalgae over protein. However, it is worth noting that the initial nitrate concentration in WP was relatively low. Overall, Nannochloropsis were able to remove approximately 87% of protein and 74% of phosphate. Statistical analysis (ANOVA) of the difference in nitrate concentration between untreated WP and algal-treated WP revealed that the observed changes were significant (p < 0.05). ANOVA analyses on protein concentration and phosphate concentration also showed statistically significant differences (p < 0.05) for the respective nutrients between untreated WP and treated WP. The protein removal efficiency reported in this study compares well with results from our previous study (Kiani et al. 2022) which showed Nannochloropsis (and associated bacterial consortium) ability to hydrolyse proteins and remove up to 81% of available proteins in dairy waste.
While ammonium consumption occurred during cultivation, the difference of ammonium concentration before and after microalgae treatment was not significant (p > 0.1). This could be attributed to the low initial ammonium concentrations in WP and/or potential ammonium production through the hydrolysis of amino acids which masked true level of ammonium removal (Kiani et al. 2022).
Cell population dynamics
Figure 2 provides insights into the cell population and cell size. The total cell counts indicate a steady increase in cell density and mean diameter for both WP and standard-medium cultures. The culture grown in the standard medium showed a noticeably higher cell density, while the cells in the culture grown on WP were observed to display a higher average diameter compared to those grown on standard medium. The results of cell population analysis suggested that biomass concentration is an aggregate of both cell density and cell size, and that the degree of contributions of either variable can differ depending on nutrient availability and culture conditions. In standard medium, the changes in biomass concentration appeared to be driven by cell density, while those in WP appeared to be driven by both cell density and cell size.
The microscopic observations were verified by cell population analysis using a flow cytometer, (Fig. 3). Flow cytometry revealed that the proportions of both large cells (or cell aggregates) and small cells increased. Conversely, in the standard medium, only the proportion of small cells increased, while large cells exhibited a decreasing trend.
Cell population dynamics of N. oceanica cultures on standard medium or WP, as measured by flow cytometry. Different types of cells including small, medium, and large cells are categorized based on side scatter vs forward scatter. Cell with high chlorophyll content (High Chl), medium chlorophyll content (Mid Chl) and low chlorophyll content (Low Chl) are distinguished based on autofluorescence. The cut-off units were arbitrary intensities given by the instrument based on preliminary gating trials of the flow cytometer. In terms of cell diameter, the upper thresholds for the intensity for small, medium and large cells corresponded to 104, 106 and 107 arbitrary forward scattered light detector units, respectively. For chlorophyll content, the thresholds for low, medium, and high chlorophyll content were 105, 106, and 107 arbitrary fluorescent light detector units (at 690 nm)
Cell size measurements by microscopy and flow cytometry demonstrated that cells cultivated on WP had significantly larger mean diameters compared to those grown on the standard medium. The values of standard deviation in the mean diameter reported in Fig. 2 indicate the distribution of diameter values within the cell population. As shown in Fig. 2, cells in WP cultures displayed a much wider spread of diameters compared to those in standard medium, implying that the cells in the WP likely existed in diverse forms ranging from small single cells to large single cells and cell aggregates/clusters. This corroborated our earlier observation using flow cytometry which showed a high proportion of large cells (or cell aggregates) in the WP cultures. Even though the discrimination between large single cells and cell aggregates was not carried out in this study, further studies can investigate this subject further using complementary methods, such as microfluidic devices, flow cytometry, image processing, and fluorescence resonance energy transfer. (Martin et al. 1997; Huerta et al. 2006; Peniuk et al. 2016; Westerwalbesloh et al. 2019; Di Caprio et al. 2021).
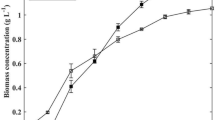
Figure 4 illustrates the biomass concentration of N. oceanica over the cultivation period, comparing biomass production on standard medium and WP. In both media, biomass concentration increased, reaching approximately 0.3 g L-1 of biomass within two weeks. Biomass concentration in the WP cultures increased slowly in the first week but exhibited an accelerated rise in the second week. WP cultures eventually caught up with the standard-medium cultures and achieved a similar final biomass concentration. Statistical analysis indicated no significant difference (p < 0.05) in final biomass concentration between WP cultures and standard-medium cultures.
Nannochloropsis displayed rapid initial growth in the standard medium, possibly due to the abundance of N in readily accessible form (i.e., nitrate). On the other hand, when cultivated on WP, the algae experienced an initial delay in biomass production prior to rapid growth during the later stage of cultivation. This pattern is aligned with the composition of the medium (Table 1), which had a high overall N content, but most of which were in an inaccessible form (i.e. proteins) that cannot be directly assimilated by the cells. In WP, the microalgal cells and their associated bacterial cells needed to first digest the proteins to provide nitrogen for their cell division, leading to a slower initial growth. However, as more nitrogen became accessible, the cells were able to compensate for the initial lag and accelerate biomass production, resulting in a similar final biomass concentration as that achieved in standard medium.
Microscopy and flow cytometry investigations
The CfW staining (Fig. 5a) indicated that both standard-medium and WP cells had a high carbohydrate content in their cell walls. In Fig. 5a, the blue-coloured regions emitting fluorescent signal represented carbohydrates and extracellular polymeric substances (EPS). In the scatter plot on the right panel, flow cytometry analyses of both WP cultures and standard-medium cultures are juxtaposed, with the green dots representing cells grown on WP and the red dots denoting cells grown on standard medium. Flow cytometry results for WP cells indicated an upward shift in forward scattered light compared to cells grown on standard medium, indicating larger cell dimensions. Large individual cells and cell aggregates are highlighted in the corresponding CfW-stained micrographs.
Cells cultivated on both standard medium and WP displayed similar levels of fluorescence when subjected to BODIPY staining (Fig. 5b), implying that both biomasses contain similar levels of intracellular lipids. The analysis also revealed that smaller cells in WP cultures exhibited a stronger intensity of BODIPY fluorescence, as indicated by the green ellipse in the scatter plot.
The autofluorescence of the cells was analysed at Ex546:Em575-640 and Ex640/30:Em690/50 to investigate effect on cellular pigment profiles (Fig. 5c and d respectively). Cells grown in standard medium displayed stronger autofluorescence compared to cells grown in WP, as indicated by an upward shift in fluorescence intensity of standard-medium cells (red dots) in the scatter plots. Since autofluorescence signals are attributed to chlorophyll, these results indicated that the WP cells contained less photosynthetic pigments compared to the standard-medium cells. The final micrograph (Fig. 5e) presents a multichannel image of the cells, with a simultaneous display of the fluorescence for all studied channels.
The brightfield micrographs of microalgae cultivated in standard medium and WP are depicted in Fig. 6. The arrows on the images signify different types of cells in the cultures. The standard-medium cultures comprised primarily of single cells, while the WP cultures were more heterogenous in terms of cellular morphologies, displaying large single cells and cell aggregates in addition to small single cells.
Fatty acid profile
The fatty-acid profiles of N. oceanica cultivated on WP and standard medium are shown in Table 2. The major fatty acids present in the lipid belonged to the C16, C18 and C20 fatty-acid families. EPA (C20:5) was detected in biomass obtained from either standard f/2 medium or WP. In biomass grown on WP, EPA accounted for 16.33% of total fatty acids which was lower than the EPA content of biomass grown on standard medium at 30.40% of total fatty acids.
Discussion
The salinity of WP was lower than seawater but higher than freshwater. The total nitrogen content in WP was higher than that in standard medium (Table 1). Protein constituted the primary form of nitrogen in WP. The level of nitrogen in free forms (i.e., as nitrate) was lower compared to standard medium. Our previous study (Kiani et al. 2022) revealed that certain microalgal species (and their associated bacteria) can use protein as their N source, but this would likely require the cells to first secrete extracellular enzymes to digest/hydrolyse protein molecules and thus result in a slower initial growth rate. The proteins present in WP can also potentially provide carbon for the biomass via mixotrophic pathway, thereby contributing to the growth of microalgae.
It is important to highlight that membrane filtration was used to generate the WP investigated in this study. Since membrane nanofiltration does not offer a perfect rejection for each compound present in the feed, minor fractions of certain compounds are expected to pass through the membrane. Membrane filtration of protein-rich dairy products (e.g., whey) therefore results in a generation of a permeate stream that is rich proteins and peptides of varying sizes. WP also displayed a notably higher phosphate content compared to the standard medium. Elevated phosphate content has previously been recognized as a factor contributing to variation in cell diameter and lipid contents (Powell et al. 2009; Simionato et al. 2013; Popko et al. 2016).
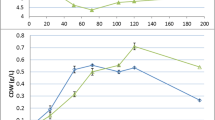
In Table 3, the chemical composition of WP and final biomass concentration obtained in this study are compared to values reported in other studies investigating microalgal capacity for wastewater treatment. Dairy wastewaters studied in the literature exhibited a wide range of N:P ratio, from 0.8 to 41.5, with total nitrogen content spanning from 10.2 to 1,492 mg L-1, and total phosphorus varying from 1 to 154 mg L-1. Fig. 7 illustrates the relationship between N and P levels with biomass productivity, categorized as low or high N:P ratios, using data extracted from Table 3. According to Huo et al. (2020), ideal values for N:P ratio to support algal growth generally lie between 5:1 to 30:1. An N:P ratio below 5:1 can lead to nitrogen limitation in cells, while a ratio exceeding 30:1 can induce phosphorus limitation. Dairy by-products and wastewater tend to have a high P content and consequently a low N:P ratio leading to nitrogen limitation (Makut et al. 2019). The value for N:P ratio of WP employed in our study was 1.47. N limitation has been demonstrated to promote lipid accumulation in microalgae in expense of biomass productivity. Even though the effect of P limitation on growth and lipid accumulation is not well established at present, several studies have demonstrated positive correlation between P deficiency in the medium and increased biomass lipid content (Simionato et al. 2013; Popko et al. 2016). It is worth noting that the ideal value for N:P ratio is also species dependent, with certain microalgae species having the capacity to store excess phosphate in cytoplasmic poly-phosphate bodies (Powell et al. 2009).
Cell population analysis (Figs. 2 and 3) showed that, in both WP and standard medium, the proportion of small-sized cells exhibited an increasing trend, indicating active cell division and proliferation. The proportion of medium-sized cells decreased for both media, suggesting ongoing cell division. Notably, the proportion of large-sized cells exhibited an increasing trend only in the WP cultures. Flow cytometry analysis with CfW staining (Fig. 5) revealed that the large-sized cells in WP are comprised of two sub-populations: a) large single cells that have likely experienced volumetric expansion due to lipid accumulation and b) large cell aggregates.
Microscopic observations (Fig. 6) confirmed that, in the standard medium, the cells exhibited uniformity in size and were typically found as single cells. However, in WP, the cells displayed a diversity of sizes and physiological states, ranging from single cells (of various sizes) to cell aggregates with distinct cell-to-cell attachment formats. Aggregate formation in microalgae is underpinned by cellular response to a range of environmental conditions (such as salinity stress and nutrient limitation) and generally involves the overproduction of extracellular polymeric substances (EPS) to glue or adhere the single cells together in a floc. In our WP cultures, aggregate formation was likely triggered through a combination of salinity stress and nitrogen depletion.
The cells in WP cultures exhibited a lower chlorophyll autofluorescence compared the cells in the standard medium. The lower cellular chlorophyll content indicates that the cells are less reliant on photosynthesis as a) they likely have other carbon sources in the WP (e.g., protein) to tap into via mixotrophic route and/or b) are nitrogen depleted due to lack of access to freely available inorganic nitrogen (as measured by chemical composition for WP in Table 1). Overall, the observed difference in autofluorescence suggested that autotrophy is the predominant metabolic activity for cells grown in standard medium, whereas both autotrophy and mixotrophy occurred in cells grown in WP.
There have been several recent studies exploring the application of dairy wastewater to cultivate microalgae. The biomass concentration achieved in this study (0.3 g L-1) is comparable to our previous study (Kiani et al. 2022) which attained a final biomass concentration of 0.3, 0.27, and 0.36 g L-1 for the cultivation of N. oceanica, Tetradesmus obliquus, and Chlorella vulgaris, respectively, on dairy wastewater within two weeks. Other studies investigating the use of microalgae for dairy wastewater treatment have reported final biomass concentrations from 0.43 to 2 g L-1 (Ummalyma and Sukumaran 2014; Daneshvar et al. 2018, 2019; Gramegna et al. 2020; Chandra et al. 2021). Biomass productivity in microalgae-mediated wastewater treatment is closely tied to nutrient availability in the wastewater and the algae species chosen for treatment. Although Nannochloropsis shows lower biomass concentrations compared to other species, it stands out as a genus rich in EPA, thus offering a unique potential to treat dairy by-products while contributing to the production of ω-3 PUFA-enriched products for valuable feed, food, and nutraceutical applications.
The native dairy medium employed in our study had a low salinity, which might have subjected the Nannochloropsis cells to hypotonic stress and thus contributed to the formation of large cell aggregates (as discussed above). However, despite this challenge, the algae demonstrated resilience, adapting to the hyposaline environment and managing to achieve a similar final biomass concentration to standard medium. Daneshvar et al. (2018) investigated the use of both freshwater (S. quadricauda) and saltwater (T. suecica) microalgal species to treat dairy wastewater and achieved a maximum biomass concentration of 0.47 and 0.61 g L-1, respectively. It is important to note that, in their study, salts were intentionally added for the marine species, potentially contributing to the higher biomass yield observed for Tetraselmis. In contrast, no additional salts were introduced to the WP medium used for growing N. oceanica in our study. This omission was a deliberate choice intended to optimise the scalability of microalgal solution in wastewater treatment. Salt addition increases algal cultivation costs, while imposing unnecessary harm to the environment by elevating the salinity of discharged water. Overall, the need for salinity amendment is a critical hurdle in the commercialisation of microalgal-based wastewater treatment for many agri-food manufacturers and research in the area should thus focus on growing microalgae in native waste without salt adjustment (when possible) in order to maintain commercial appeal.
Cultivation in WP was demonstrated to favour the production of saturated and monounsaturated fatty acids in N. oceanica, particularly C16 fatty acids (Table 3). As mentioned previously, the WP utilized in this study had a significantly lower N:P ratio compared to the standard medium. Additionally, the nitrogen present in the medium was predominantly in the form of proteins, which likely required more complex metabolic processes to assimilate and therefore were not readily accessible by the cells, thus intensifying nitrogen depletion. Several physiological indicators observed in the study, such as presence of large single cells, formation of cell aggregates, and low level of chlorophyll autofluorescence, strongly indicated that the cells were nitrogen depleted. Nitrogen depletion has consistently been proven to be an effective strategy for inducing lipid accumulation in Nannochloropsis. However, the lipids accumulated during nitrogen depletion typically manifest as neutral lipid globules, rich in saturated and monounsaturated fatty acids (Olmstead et al. 2013; Simionato et al. 2013). Since PUFAs are generally found in membrane phospholipids and glycolipids, they accumulated to a lesser extent during nitrogen starvation. The results in this study are in agreement with previous studies reporting on lipid accumulation in Nannochloropsis under different nitrogen levels, showing that the fatty acid profile of biomass grown in nitrogen-depleted WP is rich in saturated and monounsaturated fatty acids (79.51% of total fatty acids) and deficient in PUFA content (20.49% of total fatty acids) relative that grown on standard medium (Olmstead et al. 2013; Simionato et al. 2013; Lai 2015; Xiao et al. 2015).
Despite the lower PUFA content of the WP biomass relative to standard-medium biomass, the overall concentration of EPA in the microalgal lipids derived from WP cultivation is similar with fish oil (between 10 – 25% of total fatty acids), affording the lipids with potential use as feed/food ingredients (Sahena et al. 2009).
Overall, the results of this study showed that N. oceanica cells were able to grow on WP without the need for any salinity amendment, nutrient addition, and water dilution. Even though the cells initially struggled to grow on WP, they adapted to the low-salinity, nitrogen-limited, and carbon-rich environment of the medium, with WP cultures managing to achieve a similar final biomass concentration to cultures grown on a standard medium. As part of their acclimatisation, cells grown on WP displayed reduced photosynthetic activity (as measured by chlorophyll autofluorescence) and increased cell aggregation. The cells also accumulated storage lipids rich in C16:0 and C18:0 fatty acids, consistent with findings from other studies recently investigating lipid accumulation in Nannochloropsis.
Conclusion
Nano-filtered WP exhibited lower salinity and nitrate content than standard medium. The N:P ratio in WP was low (1.47) relative to standard medium, with the majority of nitrogen in protein molecules rather than existing in free forms. The medium was found to be able to support microalgal growth, enabling N. oceanica to be cultivated without salt adjustment, nutrient amendment or dilution. After initially experiencing a growth lag in the early stage of cultivation, N. oceanica adapted to WP environment and exhibited a sharper biomass increase in the later stage of cultivation, achieving a similar final biomass concentration to standard-medium cultures (ca. 0.3 g biomass L-1). The cells were able to remove 100% nitrate, 87% protein, and 74% phosphate from WP. As part of their acclimatisation mechanism, cells grown on WP displayed diverse morphological states, ranging from single cells to large cell aggregates. They also exhibited lower a photosynthetic activity (as measured by chlorophyll autofluorescence), suggestive of heightened mixotrophic activity using organic carbon sources in WP. Nitrogen limitation led to storage-lipid accumulation and WP biomass rich in C16:0 and C18:0 fatty acids. Despite this, the overall concentration of EPA in the microalgal lipids derived from WP cultivation (16.6% of total fatty acids) was still competitive to the ω-3 PUFAs found in fish oil (10 – 25% of total fatty acids), thereby validating the algal lipid’s potential application as an ω-3-lipid replacement in feed/food ingredients.
Availability of data and material
The raw data supporting the conclusions of this article will be made available by the authors upon request.
References
Adarme-Vega TC, Thomas-Hall SR, Schenk PM (2014) Towards sustainable sources for omega-3 fatty acids production. Curr Opin Biotech 26:14–18
Ahmad S, Pathak VV, Kothari R, Kumar A, Naidu Krishna SB (2018) Optimization of nutrient stress using C. pyrenoidosa for lipid and biodiesel production in integration with remediation in dairy industry wastewater using response surface methodology. 3 Biotech 8:326
Bendimerad-Benmokhtar S, Bouanane S, Merzouk H, Baba Ahmed FZ, Bendaoud A (2019) Effects of Nannochloropsis fed on serum and tissue lipids metabolism in obese offspring of overfed dams. Curr Nutr Food Sci 15:72–86
Biswas T, Bhushan S, Prajapati SK, Ray Chaudhuri S (2021) An eco-friendly strategy for dairy wastewater remediation with high lipid microalgae-bacterial biomass production. J Environ Manage 286:112196
Bryson E, Willis J (2017) Foundation mathematics for biosciences. Pearson, London
Chandra R, Pradhan S, Patel A, Ghosh UK (2021) An approach for dairy wastewater remediation using mixture of microalgae and biodiesel production for sustainable transportation. J Environ Manage 297:113210
Chen C-Y, Nagarajan D, Cheah WY (2018) Eicosapentaenoic acid production from Nannochloropsis oceanica CY2 using deep sea water in outdoor plastic-bag type photobioreactors. Bioresour Technol 253:1–7
Choi Y-K, Jang HM, Kan E (2018) Microalgal biomass and lipid production on dairy effluent using a novel microalga, Chlorella sp. isolated from dairy wastewater. Biotech Bioproc Eng 23:333–340
Chokshi K, Pancha I, Ghosh A, Mishra S (2016) Microalgal biomass generation by phycoremediation of dairy industry wastewater: an integrated approach towards sustainable biofuel production. Bioresour Technol 221:455–460
Daneshvar E, Zarrinmehr MJ, Hashtjin AM, Farhadian O, Bhatnagar A (2018) Versatile applications of freshwater and marine water microalgae in dairy wastewater treatment, lipid extraction and tetracycline biosorption. Bioresour Technol 268:523–530
Daneshvar E, Zarrinmehr MJ, Koutra E, Kornaros M, Farhadian O, Bhatnagar A (2019) Sequential cultivation of microalgae in raw and recycled dairy wastewater: microalgal growth, wastewater treatment and biochemical composition. Bioresour Technol 273:556–564
de Almeida Pires T, Cardoso V, Batista F (2021) Feasibility of Chlorella vulgaris to waste products removal from cheese whey. Int J Environ Sci Technol 19:4713–4722
Di Caprio F, Posani S, Altimari P, Concas A, Pagnanelli F (2021) Single cell analysis of microalgae and associated bacteria flora by using flow cytometry. Biotech Bioproc Eng 26:898–909
Ghobrini D, Potocar T, Smolova J, Krausova G, Yakoub-Bougdal S, Branyik T (2020) Heterotrophic cultivation of Chlorella vulgaris using saline waste water from the demineralization of cheese whey. Biotech Lett 42:209–217
Gonçalves AL, Pires JCM, Simões M (2016) Biotechnological potential of Synechocystis salina co-cultures with selected microalgae and cyanobacteria: nutrients removal, biomass and lipid production. Bioresour Technol 200:279–286
Goswami G, Makut BB, Das D (2019) Sustainable production of bio-crude oil via hydrothermal liquefaction of symbiotically grown biomass of microalgae-bacteria coupled with effective wastewater treatment. Sci Rep 9:15016
Gramegna G, Scortica A, Scafati V, Ferella F, Gurrieri L, Giovannoni M, Bassi R, Sparla F, Mattei B, Benedetti M (2020) Exploring the potential of microalgae in the recycling of dairy wastes. Bioresour Technol Rep 12:100604
Guruvaiah M, Narra M, Shah E, James J, Kurchania A (2015) Utilization of dairy wastewater for pollutants removal and high lipid biomass production by a newly isolated microalgal strains Chloromonas playfairii and Desmodesmus opoliensis. Int J Appl Sci Biotech 3:699–707
Halim R, Hill DR, Hanssen E, Webley PA, Blackburn S, Grossman AR, Posten C, Martin GJ (2019) Towards sustainable microalgal biomass processing: anaerobic induction of autolytic cell-wall self-ingestion in lipid-rich Nannochloropsis slurries. Green Chem 21:2967–2982
Huerta L, López-Balderas N, Larralde C, Lamoyi E (2006) Discriminating in vitro cell fusion from cell aggregation by flow cytometry combined with fluorescence resonance energy transfer. J Virol Meth 138:17–23
Huo S, Kong M, Zhu F, Qian J, Huang D, Chen P, Ruan R (2020) Co-culture of Chlorella and wastewater-borne bacteria in vinegar production wastewater: enhancement of nutrients removal and influence of algal biomass generation. Algal Res 45:101744
Kiani H, Azimi Y, Li Y, Mousavi M, Cara F, Mulcahy S, McDonnell H, Blanco A, Halim R (2022) Nitrogen and phosphate removal from dairy processing side-streams by monocultures or consortium of microalgae. J Biotech 361:1–11
Koreivienė J, Valčiukas R, Karosienė J, Baltrėnas P (2014) Testing of Chlorella/Scenedesmus microalgae consortia for remediation of wastewater, CO2 mitigation and algae biomass feasibility for lipid production. J Env Eng Landscape Manage 22:105–114
Kruger NJ (2009) The Bradford method for protein quantitation. Meth Mol Biol 32:9–15
Kumar AK, Sharma S, Patel A, Dixit G, Shah E (2019) Comprehensive evaluation of microalgal based dairy effluent treatment process for clean water generation and other value added products. Int J Phytoremed 21:519–530
Lai Y-J (2015) Omega-3 fatty acid obtained from Nannochloropsis oceanica cultures grown under low urea protect against Abeta-induced neural damage. J Food Sci Technol 52:2982–2989
Lee I, Han J-I (2015) Hydrothermal-acid treatment for effectual extraction of eicosapentaenoic acid (EPA)-abundant lipids from Nannochloropsis salina. Bioresour Technol 191:1–6
Lucakova S, Branyikova I, Branyik T, Matoulkova D, Krausova G (2022) Wastewater from the demineralization of cheese whey for cost-efficient cultivation of spirulina. J Appl Phycol 34:89–99
Magoni C, Bertacchi S, Giustra CM, Guzzetti L, Cozza R, Ferrari M, Torelli A, Marieschi M, Porro D, Branduardi P (2022) Could microalgae be a strategic choice for responding to the demand for omega-3 fatty acids? A European perspective. Trends Food Sci Technol 121:142–155
Makut BB, Das D, Goswami G (2019) Production of microbial biomass feedstock via co-cultivation of microalgae-bacteria consortium coupled with effective wastewater treatment: a sustainable approach. Algal Res 37:228–239
Martin I, Dozin B, Quarto R, Cancedda R, Beltrame F (1997) Computer-based technique for cell aggregation analysis and cell aggregation in in vitro chondrogenesis. Cytometry 28:141–146
Moore JW, Stanitski CL (2014) Chemistry: the molecular science, 5th edn. Cengage Learning, Boston
Oliver L, Dietrich T, Marañón I, Villarán MC, Barrio RJ (2020) Producing omega-3 polyunsaturated fatty acids: a review of sustainable sources and future trends for the EPA and DHA market. Resources 9:148
Olmstead IL, Hill DR, Dias DA, Jayasinghe NS, Callahan DL, Kentish SE, Scales PJ, Martin GJ (2013) A quantitative analysis of microalgal lipids for optimization of biodiesel and omega-3 production. Biotech Bioeng 110:2096–2104
Pal D, Khozin-Goldberg I, Cohen Z, Boussiba S (2011) The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl Microbiol Biotechnol 90:1429–1441
Pandey A, Srivastava S, Kumar S (2019) Isolation, screening and comprehensive characterization of candidate microalgae for biofuel feedstock production and dairy effluent treatment: a sustainable approach. Bioresour Technol 293:121998
Pandey A, Srivastava S, Kumar S (2020) Development and cost-benefit analysis of a novel process for biofuel production from microalgae using pre-treated high-strength fresh cheese whey wastewater. Env Sci Pollut Res 27:23963–23980
Peniuk G, Schnurr P, Allen D (2016) Identification and quantification of suspended algae and bacteria populations using flow cytometry: applications for algae biofuel and biochemical growth systems. J Appl Phycol 28:95–104
Popko J, Herrfurth C, Feussner K, Ischebeck T, Iven T, Haslam R, Hamilton M, Sayanova O, Napier J, Khozin-Goldberg I (2016) Metabolome analysis reveals betaine lipids as major source for triglyceride formation, and the accumulation of sedoheptulose during nitrogen-starvation of Phaeodactylum tricornutum. PLoS One 11:e0164673
Powell N, Shilton A, Chisti Y, Pratt S (2009) Towards a luxury uptake process via microalgae–defining the polyphosphate dynamics. Water Res 43:4207–4213
Qie F, Zhu J, Rong J, Zong B (2019) Biological removal of nitrogen oxides by microalgae, a promising strategy from nitrogen oxides to protein production. Bioresour Technol 292:122037
Qin L, Wang Z, Sun Y, Shu Q, Feng P, Zhu L, Xu J, Yuan Z (2016) Microalgae consortia cultivation in dairy wastewater to improve the potential of nutrient removal and biodiesel feedstock production. Env Sci Pollut Res 23:8379–8387
Rasouli Z, Valverde-Pérez B, D’Este M, De Francisci D, Angelidaki I (2018) Nutrient recovery from industrial wastewater as single cell protein by a co-culture of green microalgae and methanotrophs. Biochem Eng J 134:129–135
Renuka N, Sood A, Ratha SK, Prasanna R, Ahluwalia AS (2013) Evaluation of microalgal consortia for treatment of primary treated sewage effluent and biomass production. J Appl Phycol 25:1529–1537
Roleda MY, Slocombe SP, Leakey RJG, Day JG, Bell EM, Stanley MS (2013) Effects of temperature and nutrient regimes on biomass and lipid production by six oleaginous microalgae in batch culture employing a two-phase cultivation strategy. Bioresour Technol 129:439–449
Sahena F, Zaidul I, Jinap S, Saari N, Jahurul H, Abbas K, Norulaini N (2009) PUFAs in fish: extraction, fractionation, importance in health. Compr Rev Food Sci Food Safety 8:59–74
Sánchez-Zurano A, Rodríguez-Miranda E, Guzmán JL, Acién-Fernández FG, Fernández-Sevilla JM, Molina Grima E (2021) Abaco: a new model of microalgae-bacteria consortia for biological treatment of wastewaters. Appl Sci 11:998
Shahid A, Malik S, Zhu H, Xu J, Nawaz MZ, Nawaz S, Alam MA, Mehmood MA (2020) Cultivating microalgae in wastewater for biomass production, pollutant removal, and atmospheric carbon mitigation; a review. Sci Total Environ 704:135303
Shetty P, Boboescu IZ, Pap B, Wirth R, Kovács KL, Bíró T, Futó Z, White Iii RA, Maróti G (2019) Exploitation of algal-bacterial consortia in combined biohydrogen generation and wastewater treatment. Front Energy Res 7:52
Silva-Benavides AM, Torzillo G (2012) Nitrogen and phosphorus removal through laboratory batch cultures of microalga Chlorella vulgaris and cyanobacterium Planktothrix isothrix grown as monoalgal and as co-cultures. J Appl Phycol 24:267–276
Simionato D, Block MA, La Rocca N, Jouhet J, Maréchal E, Finazzi G, Morosinotto T (2013) The response of Nannochloropsis gaditana to nitrogen starvation includes de novo biosynthesis of triacylglycerols, a decrease of chloroplast galactolipids, and reorganization of the photosynthetic apparatus. Eukaryotic Cell 12:665–676
Singh RB, Choudhury J, De Meester F, Wilczynska A, Dharwadkar S, Wilson D (2012) Association of high W-6/W-3 ratio paleolithic style diets and risk of cardiovascular diseases and other chronic diseases: is the tissue the main issue? World Heart J 4:189
Su Y, Mennerich A, Urban B (2012) Coupled nutrient removal and biomass production with mixed algal culture: impact of biotic and abiotic factors. Bioresour Technol 118:469–476
Tripathi BD, Shukla SC (1991) Biological treatment of wastewater by selected aquatic plants. Env Pollut 69:69–78
Udaiyappan AFM, Hasan HA, Takriff MS, Abdullah SRS, Maeda T, Mustapha NA, Yasin NHM, Hakimi NINM (2020) Microalgae-bacteria interaction in palm oil mill effluent treatment. J Water Proc Eng 35:101203
Ummalyma SB, Sukumaran RK (2014) Cultivation of microalgae in dairy effluent for oil production and removal of organic pollution load. Bioresour Technol 165:295–301
Verveniotis A, Siasos G, Oikonomou E, Tsigkou V, Papageorgiou N, Zaromitidou M, Psaltopoulou T, Marinos G, Deftereos S, Vavuranakis M (2018) The impact of omega 3 fatty acids in atherosclerosis and arterial stiffness: an overview of their actions. Curr Pharmaceut Design 24:1865–1872
Wan C, Bai F-W, Zhao X-Q (2013) Effects of nitrogen concentration and media replacement on cell growth and lipid production of oleaginous marine microalga Nannochloropsis oceanica DUT01. Biochem Eng J 78:32–38
Wani AL, Bhat SA, Ara A (2015) Omega-3 fatty acids and the treatment of depression: a review of scientific evidence. Integr Med Res 4:132–141
Westerwalbesloh C, Brehl C, Weber S, Probst C, Widzgowski J, Grünberger A, Pfaff C, Nedbal L, Kohlheyer D (2019) A microfluidic photobioreactor for simultaneous observation and cultivation of single microalgal cells or cell aggregates. PLoS One 14:e0216093
Wilkie AC, Mulbry WW (2002) Recovery of dairy manure nutrients by benthic freshwater algae. Bioresour Technol 84:81–91
Xiao Y, Zhang J, Cui J, Feng Y, Cui Q (2013) Metabolic profiles of Nannochloropsis oceanica IMET1 under nitrogen-deficiency stress. Bioresour Technol 130:731–738
Xiao Y, Zhang J, Cui J, Yao X, Sun Z, Feng Y, Cui Q (2015) Simultaneous accumulation of neutral lipids and biomass in Nannochloropsis oceanica IMET1 under high light intensity and nitrogen replete conditions. Algal Res 11:55–62
You K, Ge F, Wu X, Song K, Yang Z, Zhang Q, Liu Y, Ruan R, Zheng H (2021) Nutrients recovery from piggery wastewater and starch wastewater via microalgae-bacteria consortia. Algal Res 60:102551
Zárate R, el Jaber-Vazdekis N, Tejera N, Pérez JA, Rodríguez C (2017) Significance of long chain polyunsaturated fatty acids in human health. Clin Translat Med 6:25
Zhang L, Ye S-C, Chen W-B, Han J-C, Tian J-J, Zhang Y-B, Xu J-L, Cao J-Y, Qin C (2021) Screening the rate-limiting genes in the ω6 polyunsaturated fatty acid biosynthesis pathway in Nannochloropsis oceanica. Algal Res 57:102342
Zkeri E, Iliopoulou A, Katsara A, Korda A, Aloupi M, Gatidou G, Fountoulakis MS, Stasinakis AS (2021) Comparing the use of a two-stage MBBR system with a methanogenic MBBR coupled with a microalgae reactor for medium-strength dairy wastewater treatment. Bioresour Technol 323:124629
Acknowledgements
Hossein Kiani would like to acknowledge Enterprise Ireland and the European Union’s Horizon 2020 Research and Innovation Programme under the Marie Skłodowska-Curie grant agreement No 847402 (Co-funding of regional, national, and international programmes, Project ID: MF 2020 0108) for their financial support. Yuchen Li would like to acknowledge the University College Dublin-China Scholarship Council (UCD-CSC) Programme. Qinge Ma and Felix Joel Brooke acknowledge the Department of Agriculture, Food and the Marine (DAFM) under the 2021 trans-European Era-Net Susfood/FOSC programme (Project ID: 2021SUSFOODFOSCEN101) for financial support. Svitlana Miros thanks the Irish Research Council (URS/2022/3L) for their financial support.
Funding
Open Access funding provided by the IReL Consortium. Enterprise Ireland and the European Union’s Horizon 2020 Research and innovation Programme under the Marie Skłodowska-Curie Co-funding of regional, national, and international programmes (Project ID: MF 2020 0108) grant agreement No 847402.
Author information
Authors and Affiliations
Contributions
Hossein Kiani: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Funding acquisition, Writing; Qinge Ma: Methodology, Investigation; Mengsong Xiao: Investigation; Yuchen Li: Methodology; Felix Joel Brooke: Investigation; Shane Mulcahy: Review & editing, Funding acquisition; Svitlana Miros: Methodology; Ronald Halim: Conceptualization, Methodology, Resources, Review & editing, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kiani, H., Ma, Q., Xiao, M. et al. Growth and fatty acid profile of Nannochloropsis oceanica cultivated on nano-filtered whey permeate. J Appl Phycol (2024). https://doi.org/10.1007/s10811-024-03287-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10811-024-03287-x