Abstract
Biofluorescence in certain seaweed species has been studied in association with their respective chlorophyll type and concentration. However, there is a paucity of information on the biofluorescent signature of macroalgal species at different life stages. Here we document that hyperspectral imaging differentiates between the fluorescent signatures of 5 macroalgal species (Ascophyllum nodosum, Fucus vesiculosus, Laminaria digitata, Palmaria palmata, and Saccharina latissima) collected from Tromsø, northern Norway (69-70 °N). Non-reproductive (NR) blades were collected for all species and reproductively mature blades (R) with sori were collected for three species. Hyperspectral imaging of collected blades was conducted under excitation lighting (~445 nm). We further assessed whether biofluorescence is reflected in the reproductive phenology of three selected species, L. digitata, P. palmata, and S. latissima. The spectral radiance of fluorescence emissions was found to be consistently different between R and NR blades. The greatest difference between R and NR blades was between the rhodophyte P. palmata (0.0075 W nm-1sr-1m-2 at 725 nm) followed by the phaeophytes L. digitata (0.03 W nm-1sr-1m-2 at 730-740 nm) and S. latissima (0.00225 W nm-1sr-1m-2 at 735-745 nm). Biofluorescence monitoring with hyperspectral imaging should be considered for further applicability in commercial seaweed operations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biofluorescence is the production of fluorescent light emissions in the tissue of living organisms at a longer wavelength than the absorbed environmental light (Maxwell & Johnson 2000). Changes within chlorophyll produced fluorescence in plants were observed as early as 1960 (Kautsky 1960; Maxwell & Johnson 2000) . Biofluorescence in macroalgae is well documented and is primarily associated with the type and concentration of photosynthetic pigments such as chlorophyll-a and accessory pigments, like phycobiliproteins and xanthophylls (Huot et al. 2018). Chlorophyll is well known for emitting a characteristic red fluorescence in macroalgae. However, other compounds present within the tissue of macroalgae fluoresce, including the photosynthetic accessory pigment phycoerythrin. The intensity and spectral properties of biofluorescence in macroalgae can vary depending on factors like species, environmental conditions, and physiological state of the specimen. A good example is when phycoerythrin present within stressed macroalgae becomes highly fluorescent as the amount of energy a chlorophyll can direct from the photons present in the water column is reduced (Forster 2012). This effect is most applied in plant stress measurements as Fv/Fm which is a normalized ratio created by dividing variable fluorescence (Fv) by maximum fluorescence (Fm). Commercial seaweed production employs total chlorophyll fluorescence and spectrophotometry as a proxy to monitor seaweed biomass (Calmes et al. 2020) . However, determining the sexual maturity of macroalgae fronds during the reproductive stage still requires an experienced eye. This is time-consuming, subjective, or routinely requires destructive sampling.
Sexual reproduction via the production of sporelings (juvenile algae) is a critical point for viable, scalable, and sustainable production of macroalgae. In this study, we explore the potential of hyperspectral imaging of fluorescence as a non-invasive, rapid, easy to use, and reliable reproductive monitoring tool for macroalgae species. We hypothesize that each seaweed species produces a unique spectral signature (tested on five macroalgae species) while the sori (reproductive zone) of three seaweed species produces a fluorescence emission that differs from the surrounding non-reproductive tissue. By using state-of-the-art imaging techniques, we aim to assess the specificity of fluorescence as a reproductive indicator in three macroalgae species.
Materials and methods
Seaweed collection
A total of five species were collected In Tromsø, Norway during the winter 2022/2023 for analysis. The samples of non-reproductive (n= 10) and when available reproductive blades of Ascophyllum nodosum, Fucus vesiculosus, Laminaria digitata, and Palmaria palmata were collected from the macroalgal communites at the Kårvika and Harbour sites (Fig. 1) in November 2022 (Table 1). Sampling was conducted from a floating pier and rocky intertidal areas typical of the littoral zone in the two sample sites. Samples of S. latissima were collected at the Kraknes site (Fig. 1). The seaweed samples were double bagged with site seawater and transported in Styrofoam boxes to the Nofima facilities in Tromsø, Norway. Prior to imaging, all collected macroalgae were checked for heavy epiphytic growth and reproductive tissues (sori).
Imaging
Hyperspectral imaging utilized the Hyspex VNIR-1800 hyperspectral camera (Norsk Elektro Optikk AS, Norway) with a wavelength documentation capacity from 400 to 1,000 nm. Hyperspectral imaging took place within a dark room. Macroalgae were continuously recorded on an automated conveyor belt illuminated with royal blue excitation lighting (~445 nm) produced by a full spectrum LED lighting (G5 XR30 Pro Radion, Ecotech, USA). The camera was mounted 1 m above the belt with a field view of 300 mm. The spatial resolution across the track was 0.17 mm, with 1800 pixels across the width of the conveyor belt. The images were spatially binned in the across track direction to produce square pixels with a resolution of 0.34 mm.
During red-green-blue (RGB) illustrative photography sessions, representative samples of the macroalgae species were placed on a black background in a seawater filled photographic aquarium (5 liter) constructed of optic white glass. A digital single lens reflex (DSLR) camera (D5100, /Nikon AF-S 60 mm f/2.8G IF-ED Micro lens, Nikon, USA) was used for photographing macroalgae illuminated under full spectrum LED lighting used for hyperspectral imaging. The ambient light photographs were taken with cool white light. A yellow barrier filter (62DY15 62 mm 12 Filter, Tiffen, USA) was utilized to filter blue light when capturing RGB.
Hyperspectral analysis
All raw data files collected during hyperspectral imaging were radiometrically calibrated within HySpex Rad v2.5 (Norsk Elektro Optikk, Norway) to produce spectral radiance value units of \(W{m}^{-2} s{r}^{-1} n{m}^{-1}\). The calibrated files were uploaded into Breeze hyperspectral imaging software (Prediktera, Umeå, Sweden) for analysis. An average spectrum was calculated for each frond and used to make a PCA model to study the clustering of the data and which parts of the spectrum contributed the most to the variation.
Results
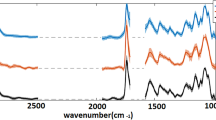
The spectral radiance of fluorescence emissions varied between species (Fig. 2) as well as between the reproductive and non-reproductive blades of the species tested (Fig. 3). Spectral analysis of the five species of sampled macroalgae found a unique fluorescence emission with an initial smaller peak (~680 nm) followed by a dominant broad emission peak (~725) in each species. Reproductive fronds have a smaller peak around 680 nm relative to the broad peak at 725 nm. The non-reproductive fronds have a taller peak at 680 nm and the broad peak is slightly shifted towards higher wavelengths (ca 735 nm). The greatest difference in R and NR was between the rhodophyte P. palmata (0.0075 W/nm sr m2/725 nm) and the phaeophytes L. digitata (0.03 W/nm sr m2/730-740 nm) and S. latissima (0.00225 W/nm sr m2 @735-745 nm).
Results of hyperspectral analysis between reproductive versus non-reproductive fronds in Laminaria digitata (A), Saccharina latissima (B), and Palmaria palmata (C). Reproductive fronds have a smaller peak around 680 nm relative to the broad peak at 725 nm. The non-reproductive have a taller peak at 680 and the broad peak is slightly shifted towards higher wavelengths (ca 735)
The spectral variation can be interpreted by using principal component analysis (PCA) to change the coordinate system so that most of the variation is represented in fewer dimensions, i.e., the principal components. This allows many-dimensional data to be shown in a 2-dimensional scatter plot without losing most of the variation in the data. A loading plot shows how the data is transformed to achieve this and which variables (in this case wavelengths) exert the most influence on the transformation.
The clustering of reproductive and non-reproductive blades in Fig. 4 shows an overlapping of spectral data on an interspecies level but not between reproductive blades on an intraspecific level. One can for instance note that non-reproductive S. latissima are spectrally similar to L. digitata, while its reproductive counterpart is in a cluster of its own. The loading plot in panel b shows that the wavelengths between 670 nm and 750 nm contribute the most to the variation in the data (97.31 % along the first principal component). The peaks and troughs suggest what the fluorescence signature of the fluorophores involved might look like.
The clustering of reproductive and non-reproductive blades in Panel A shows an overlapping of spectral data on an interspecies level but not between reproductive blades on an intraspecies level. Panel B shows the loading vectors of the components where wavelengths around 670 nm and 750 nm contribute the most to the variation in the data
Fluorescence emission produced by the five macroalgae species may be seen in Fig. 5.
Macroalgae species (top down): Fucus vesiculosus, Laminaria digitata, Palmaria palmata, Saccharina latissima, and Ascophyllum nodosum photographed for illustrative purposes under normal lighting [a] and royal blue excitation lighting (445 nm) [b]. Note the fluorescent exudate in the water column in column B of L. digitata and P. palmata photos
Discussion
This study found that hyperspectral imaging can detect the differences in fluorescence spectra produced by each of the five respective species of macroalgae tested. In addition, hyperspectral imaging can be used as a non-destructive method for differentiating between R and NR tissue of macroalgal species via their fluorescence signals. Consistent changes were noted in the fluorescence between the R and NR blades of L. digitata, P. palmata, and S. latissimia collected from the same geographic area. The low number of reproductive blades of L. digitata (n=2) should be considered when compared to the other two species (n=10). The small difference in emission spectra from the non-reproductive blades may be explained by either the small sample size available for Laminaria reproductive blades or the limited surface area occupied by sori. However, the difference in emission intensity between R and NR blade tissue shows that fluorescence signals in reproductive tissue are consistently lower in all samples. The change in the spectral shift in the fluorescence between reproductive and non-reproductive blade tissue is likely caused by shift in the transmission of light by pigments that are fluorophores, the molecules responsible for fluorescence. Known fluorophores in macroalgae include chlorophyll and accessory pigments such as phycoerythrin. Shifting amounts of light being transmitted from these fluorophores in maturing reproductive tissue could be caused by a shift in concentration, distribution, or composition of these fluorophores.
The fluorescence in seaweed is associated with chlorophyll function. The chlorophyll present within the developing reproductive tissue (sporangia) on the blade surface may be influenced through restricting light. Reproductive tissue is typically darker and slightly raised compared to the surrounding blade tissue. However, individual seaweeds and their sporangia can vary remarkedly in both appearance and structure such as that observed in our study. Although all Phaeophytes, the difference between the fluorescence spectra produced by the Fucaceae A. nodosum and F. vesiculosus is not clearly distinguishable in contrast to the signal emitted by the Laminariaceae L. digitata and S. latissima. The external fluorescence captured by hyperspectral imaging found clear differences between the latter two species. This study also finds that variation in macroalgae fluorescence exists between brown and red seaweed phyla. Such differences in fluorescence were even observed in RGB photographs (Fig. 5). However, members of both phyla (L. digitata and P. palmata) were observed producing a fluorescent exudate in the water column. This variation within seaweed phyla is supported by Huot et. al. (2018) who found differential absorption of light by the accessory pigments of three Arctic macroalgae species related to accessory pigments (i.e., Ulva sp.: chlorophyll-b; Agarum cribosum: fucoxanthin; Phycodrys rubens: phycoerythrin). This is also reflected in photosynthetic efficiency fluorescence measurements Fv/Fm in phaeophytes and rhodophytes (Lubsch & Timmermans 2019, 2020) , where the rhodophyte P. palmata: (0.61±0.04) showed a significantly lower average than the phaeophytes L. digitata (0.74±0.06) and S. latissima (0.78±0.04) in optimal cultivation conditions.
Furthermore, environmental conditions, natural variations present within population(s), and the specific geographic location(s) may also further influence spectral plasticity of macroalgal fluorescence. Macroalgae are continuously exposed to environmental changes including shifts in the irradiation spectra (Bhagooli et al. 2021). The distinct differences in pigments produced by macroalgae in response to their environment will likely influence the spectral plasticity of macroalgae fluorescence (Gevaert et al. 2002). It is well documented that variations in populations continue to influence the biochemical composition within European phaeophytes (Manns et al. 2017). For example, different geographic populations of the fucoid algae Ascophyllum nodosum do not elevate their phlorotannin levels uniformly in response to herbivory by the snail Littorina obtusata (Long & Trussell 2007). Macroalgal fluorescence measurements should consider these variabilities when producing fluorescence emission averages for specific studies.
The results of this study show that the fluorescence signals produced by macroalgae can be detected with a specificity in a nondestructive and expedient manner. The use of fluorescence measurements (Fv/Fm) has already been used for microalgae (Kromkamp & Peene 1999), seaweeds (Lubsch & Timmermans 2019, 2020) and corals (Wiedenmann et al. 2013) to monitor stress related disorders. Measuring fluorescence with hyperspectral imaging not only has the potential to address spectral plasticity in macroalgae regarding their environment and physiological state, but also has the potential to act as a proxy for reproductive phenology. This is important as the harvest of sporophytes is often affected by the poorly timed harvest of mature spores (sori), which can easily create bottlenecks in industrial scale propagation. The reliable identification and subsequent culture of seaweed blades entering their reproductive state would assist industrial operations to better scale their grow out operations in response to market demand(s). Such stabilization would help reduce the supply chain fluctuations that are a recurring issue in the current market. This study proposes extending the array of applications using fluorescence measurements in macroalgae as a reliable, easy to use, non-destructive, and quick diagnostic tool to assess reproductive maturation (sori). Further research is required to expand on the data collected within this baseline study and to explore possible intraspecies differences related to environmental, geographic, and temporal factors.
Data availability
Data collected for this experiment is available upon request to the authors.
Code availability
Code is available upon request to the authors.
References
Bhagooli R, Mattan-Moorgawa S, Kaullysing D, Louis YD, Gopeechund A, Ramah S, Soondur M, Pilly SS, Beesoo R, Wijayanti DP, Bachok ZB, Monrás VC, Casareto BE, Suzuki Y, Baker AC (2021) Chlorophyll fluorescence - A tool to assess photosynthetic performance and stress photophysiology in symbiotic marine invertebrates and seaplants. Mar Pollut Bull 165:112059
Calmes B, Strittmatter M, Jacquemin B, Perrineau M-M, Rousseau C, Badis Y, Cock JM, Destombe C, Valero M, Gachon CMM (2020) Parallelisable non-invasive biomass, fitness and growth measurement of macroalgae and other protists with nephelometry. Algal Res 46:101762
Forster R (2012) Re: Does anybody know what may cause bright “fluorescent” colouration among the drifting red algae? . https://www.researchgate.net/post/Does_anybody_know_what_may_cause_bright_fluorescent_colouration_among_the_drifting_red_algae/50ad3d79e4f0768a3e00001b/citation/download. Accessed 25 Aug 2023
Gevaert F, Créach A, Davoult D, Holl A-C, Seuront L, Lemoine Y (2002) Photo-inhibition and seasonal photosynthetic performance of the seaweed Laminaria saccharina during a simulated tidal cycle: chlorophyll fluorescence measurements and pigment analysis. Plant Cell Environ 25:859–872
Huot M, Rehm E, Dalgleish F, Piché M, Lambert-Girard S, Archambault P (2018) Characterizing fluorescence and reflectance properties of Arctic macroalgae as future LiDAR targets. Proc SPIE 10631, Ocean Sensing and Monitoring X. pp 265–273
Kautsky H (1960) Chlorophyll-fluorescenz und Kohlensaureassimilation. XIII. Mitteilung. Die Fluorescenzkurve und die Photochemie der Pflanze. Biochem Z 332:277–292
Kromkamp J, Peene J (1999) Estimation of phytoplankton photosynthesis and nutrient limitation in the Eastern Scheldt estuary using variable fluorescence. Aquat Ecol 33:101-104
Long JD, Trussell GC (2007) Geographic variation in seaweed induced responses to herbivory. Mar Ecol Prog Ser 333:75–80
Lubsch A, Timmermans KR (2020) Phosphate and nitrate uptake dynamics in Palmaria palmata (Rhodophyceae): Ecological and physiological aspects of nutrient availability. J Phycol 56:1184–1195
Lubsch A, Timmermans KR (2019) Uptake kinetics and storage capacity of dissolved inorganic phosphorus and corresponding dissolved inorganic nitrate uptake in Saccharina latissima and Laminaria digitata (Phaeophyceae). J Phycol 55:637–650
Manns D, Nielsen MM, Bruhn A, Saake B, Meyer AS (2017) Compositional variations of brown seaweeds Laminaria digitata and Saccharina latissima in Danish waters. J Appl Phycol 29:1493–1506
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
Wiedenmann J, D’Angelo C, Smith EG, Hunt AN, Legiret F-E, Postle AD, Achterberg EP (2013) Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nat Clim Chang 3:160–164
Funding
Open Access funding provided by the IReL Consortium This study was supported by the Nofima strategic programs: DeepVision and MacroALGAqua. The authors are grateful for the funding from the EU H2020 project AquaVitae (project number 818173). Further funding was received from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 956697.
Author information
Authors and Affiliations
Contributions
Conceptualization, TJD; Methodology TJD, XW, SL; Data/analysis, TJD, SL; Original draft: TJD; Seaweed specimens: XW; Review and editing, PJ, SL, XW; Supervision, PJ; Funding acquisition, PJ. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflicts of interest
The authors declare they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Juhasz-Dora, T., Lindberg, SK., James, P. et al. Assessing the potential of fluorescence as a monitoring tool for reproductive tissue in selected macroalgae species. J Appl Phycol (2024). https://doi.org/10.1007/s10811-024-03211-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10811-024-03211-3