Abstract
Species of the red algal genus Meristotheca are important natural resources that can be used directly as food for human consumption as well as raw materials for the extraction of carrageenan. Despite being harvested in Japan and Korea, a comprehensive taxonomic study of Meristotheca specimens from these two countries to elucidate their phylogenetic position is lacking. In this study, we aimed to clarify the taxonomic identities of specimens currently regarded as M. papulosa from Korea and Japan by analyzing morphological and molecular data. As the result, we recognized a new species, Meristotheca pilulaora sp. nov. from Korea and resurrected Meristotheca japonica for specimens from Japan. The two entities have long been identified as M. papulosa and exhibit similar external morphologies. They appeared independently in the concatenated phylogenetic tree of COI-5P and rbcL and could also be distinguished morphologically by the position of cystocarps (blade margins in M. pilulaora; marginal proliferations in M. japonica), spinose cystocarps (absent in M. pilulaora; present in M. japonica), and the number of cortical cell layers (two in M. pilulaora; 4–8 in M. japonica). The DNA analysis of M. pilulaora sp. nov. in local populations showed 11 COI-5P haplotypes on Jeju Island with no apparent geographical structure. High genetic diversity and occurrence of unique haplotypes in southern Jeju may provide a genetic basis for population with high thermal tolerance. These findings have strengthened our understanding of the species diversity of the genus Meristotheca and provided insights into conservation, management, and haplotype selection for the future cultivation of these economically important species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The recognition of macroalgal diversity and biogeography is relatively poor, even in economically important algae that have received considerable attention in the industrial perspective. Accurate species identification is an important issue in extraction of natural compound industrially because this was related to the reproducibility and reliability of the results (Leal et al. 2016). Molecular analyses have helped discriminate between macroalgal species and provide information on their distribution (Yang and Kim 2018; Yang et al. 2021a, b). In addition, studies on the genetic diversity of species are useful for understanding species adaptations related to temporal and spatial maintenance, particularly for the sustainable use of economic algae (Hu et al. 2015; Liang et al. 2022).
Species of Meristotheca J. Agardh are important natural resources for seaweed salad in Asia (Xia and Abbott 1987; Shinmura and Tanaka 2008; Borlongan et al. 2021), traditional food in the form of jelly (N'Yeurt 1995), and feed for abalone (Alcantara and Noro 2005). Meristotheca species are also current and potential sources of carrageenan, derived from Meristotheca procumbens P.W. Gabrielson & Kraft (Prasad et al. 2003), Japanese and South Korean M. papulosa (Montagne) J. Agardh (Fujiki and Kikutani 1977; Usov et al. 2001), M. halymenioides (Zanardini) G.W. Saunders & Kraft (Watt et al. 2003 as M. papulosa), and M. gelidium (J. Agardh) E.J. Faye & M. Masuda (Faria-Tischer et al. 2006 as Meristiella gelidium). Despite the possibility of available sources with biological activities, only natural populations of Meristotheca have been exploited for commercial use (Borlongan et al. 2021). The growth of Meristotheca species has been studied in experimental tank culture (Kimura 1992; Ohno et al. 2002), as well as callus induction and thallus regeneration (Huang and Fujita 1997), and photosynthetic responses (Lideman et al. 2012; Borlongan et al. 2020), to develop cultivation technologies for this economically important genus.
Meristotheca papulosa is the lectotype species of the genus with a type locality in Hodeida, Yemen (Montagne 1850, see Fig. 27 as type specimen in Kylin 1932). This species has been regarded as the most popular species for commercial use in the genus (Lideman et al. 2012; Borlongan et al. 2021). The most productive region of M. papulosa is Kagoshima in Japan (Ohno 2004; Shinmura and Tanaka 2008), where annual biomass production reached 440 t in 2000, with a value of approximately US$ 2,300,000 (Lideman et al. 2012, no available recent data in Japan). Meristotheca papulosa was also harvested from Jeju Island in Korea and the products from Jeju Island were exported to Japan at a higher price than other edible seaweeds (Kim et al. 2019). However, the natural populations of M. papulosa have been steadily decreasing in Korea and Japan owing to excessive harvesting and habitat loss (Lideman et al. 2012), resulting in a near-threatened species designation by the Japanese Ministry of Environment (Red List 4th edition in 2015, 5th edition in 2020) and it has not been harvested in Jeju since 2007 (Kim et al. 2019). Moreover, climate warming has altered the geographic distribution of macroalgae, greatly affecting their conservation and sustainable utilization (Zhou et al. 2023). Accordingly, appropriate management of Meristotheca based on an understanding of its species diversity and biogeography is essential.
Several studies have attempted to accurately elucidate the species diversity within the genus Meristotheca using phylogenetic methods (Faye et al. 2004, 2007, 2008; Schneider et al. 2020; Nguyen et al. 2022). Faye et al. (2004) subsumed the genus Meristiella D.P. Cheney under Meristotheca, proposing the new combination Meristotheca gelidium and the new species Meristotheca dakarensis Faye & Masuda. Faye et al. (2007, 2008) provided molecular and morphological data to clarify the taxonomic status of two Meristotheca species from Japan, M. coacta Okamura, and M. imbricata E.F. Faye & M. Masuda, and suggested that further analysis is required for specimens with similar morphological features. The distribution of M. papulosa has been questioned as it has been reported from various localities based solely on morphological identification, from Korea (Lee 2008; Kim et al. 2022), Japan (Faye et al. 2005), China (Liu 2008), Taiwan (Lewis and Norris 1987), the Philippines (Ang et al. 2014), Indonesia (Verheij and Prud'homme van Reine 1993), Pakistan (Silva et al. 1996), India (Rao and Gupta 2015), South Africa (De Clerck et al. 2005), Madagascar (Vieira et al. 2021), and Red Sea (Einav et al. 2021). On the other hand, the genetic status of Merostptjeca furgusonii Grunow ex Mazza, from Sri Lanka, Meristotheca tobagensis W.R. Talor from the Caribbean island of Tobago, and Meristotheca polychotoma (Kützing) Millar from New Caledonia required confirmation based on the reproductive features (Faye et al. 2007, 2008; Borlongan et al. 2021). A recent taxonomic study of Vietnamese specimens identified as ‘M. papulosa’ resulted in the recognition of the new species Meristotheca lysonensis X.-V. Nguyen, X.-T. Nguyen, Kittle & McDermid (Nguyen et al. 2022). Meristotheca halymenioides has been confirmed as a distinct species for specimens previously referred to M. papulosa in eastern Australia (Schneider et al. 2020). These results suggest that the species diversity at local scales within Meristotheca may be underestimated and the species distribution of M. papulosa may be overestimated.
Currently, two types of blades have been reported in Korea and Japan: erect in M. papulosa and prostrate in M. coacta and M. imbricata (Faye et al. 2005; Lee 2008; Kim et al. 2022). Faye et al. (2005) documented the vegetative and reproductive morphologies of Japanese M. papulosa, with rbcL sequence analysis. However, they questioned the phylogenetic position of Japanese M. papulosa due to the absence of comparable sequence data from the type locality. Studies on the growth and maturation patterns of M. papulosa in Korea have been conducted to provide effective data for the management and mass culture of this species (Kim et al. 2019). Nevertheless, the taxonomic position of Korean M. papulosa remains unclear without a molecular assessment. Given the economic importance of Meristotheca species, we aimed to elucidate the taxonomy and phylogenetic relationships of specimens referred to M. papulosa from Korea and Japan and to assess the intraspecific genetic diversity to contribute to the conservation and sustainable use of these important macroalgae.
Materials and methods
Sample collections and observations
A total of 118 samples of Meristotheca were collected from 16 locations in Jeju, Korea, and six locations in Japan (Table S1). Intertidal sampling was conducted during low tide, whereas subtidal sampling was performed using SCUBA diving. A portion of the fronds was excised from each plant and desiccated in silica gel for molecular analysis. For morphological observations, specimens were preserved in 5% formalin. Fresh specimens were pressed onto herbarium sheets and deposited in the herbarium of Jeju National University (JNUB), Jeju, Korea.
Microscopic cross-sections were made using a benchtop freezing microtome (NK-101-II; Nippon Optical Works Co., Ltd., Japan), stained with 1% aniline blue acidified with 1N HCl, and mounted in a 25% corn syrup/distilled water solution. Micrographs were acquired using a BX43 microscope (Olympus). Photographs of specimens were captured using an EOS 600D digital camera (Canon, Japan). Morphological measurements were obtained from micrographs using SigmaScanPro automated image analysis software (Jandel Scientific, USA). Digitized images were adjusted for clarity using Adobe Photoshop software (Adobe Systems Inc., USA; ver. 6.1).
DNA extraction, amplification, and sequencing
Genomic DNA was extracted using a MagPurix DNA Isolation Kit (Zinext, Taiwan), according to the manufacturer’s instructions. The extracted DNA was stored at ‒20 ℃ and underwent polymerases chain reaction (PCR) amplification using AccuPower PCR Premix (Bioneer, Korea) in final volume of 20 μL. Targeted sequences of the COI-5P and rbcL gene were amplified and sequenced using the primers GazF2 and GazR1 for COI-5P (Saunders 2005; Lane et al. 2007), and F7–R898 and F762–R1442 for rbcL (Kim et al. 2010). These markers were selected based on the number of sequences of Meristotheca available in GenBank for comparison with our specimens. PCR conditions were as described by Yang et al. (2021a, b). The PCR products were visually checked on a 1% agarose gel, purified using ExoSAP-IT (USB, USA), and commercially sequenced by Macrogen (Seoul, Korea). The same set of primers was used for sequencing.
Alignment, genetic diversity, and haplotype analyses
All successful amplifications of the COI-5P and rbcL genes were sequenced in both directions. Assembly and manual editing were performed using Geneious Prime v. 2022.2.2 (http://www.geneious.com). All the edited sequences were combined with available GenBank sequences and aligned using the MUSCLE algorithm in Geneious. To evaluate the relationships among COI-5P haplotypes, a haplotype network was constructed using a minimum spanning network in Arlequin ver. 3.5.2 (Excoffier et al. 2005). Uncorrected p-distances implemented in MEGA X (Kumar et al. 2018) were used to calculate genetic divergence in terms of pairwise differences between and within the species.
Phylogenetic analyses
We analyzed the COI-5P and rbcL datasets separately and concatenated the sequences to obtain a combined COI-5P-rbcL dataset. In the combined COI-5P-rbcL dataset, taxa for which sequences were unavailable were treated as missing. For the phylogenetic tree reconstruction, Tepoztequiella rhizoidea, Tepoztequiella muriamans, and Sarcodiotheca furcata were included as outgroups (Schneider et al. 2020).
Phylogenetic analyses using maximum likelihood (ML) were performed using the GTR substitution model in Geneious. Support for each branch was obtained from 1000 bootstrap replications.
Results
Morphological observations and molecular analyses revealed that the specimens from Korea and Japan did not accord with authentic M. papulosa. For the Korean ‘M. papulosa’, we propose a new species M. pilulaora M.Y.Yang & M.S.Kim, sp. nov. The Japanese ‘M. papulosa’ was distinguished from not only authentic M. papulosa but also the new Korean species; M. japonica Kylin is the earliest available name that has been applied to this species and is resurrected here.
Taxonomic treatments
Meristotheca pilulaora M.Y. Yang & M.S. Kim sp. nov. (Figs 1, 2)
Holotype. JNUB – MP168 (tetrasporophyte) was collected from the subtidal zone on August 18, 2022. GenBank accession Number: OR726878 for COI-5P; OR726850 for rbcL.
Meristotheca pilulaora M.Y. Yang & M.S. Kim sp. nov. a Fluorescent thallus in underwater,situ. b Holotype specimen (tetrasporophyte) collected from Sangmo-ri, Daejeong, Jeju, Korea on August 18, 2022. Scale bar = 5 cm, c Dried specimen of female gametophyte with cystocarps collected from Udo Strait, Jeju, Korea on August 1, 2015. Scale bar = 5 cm. d Wet specimen of vegetative thallus collected from Udo, Jeju, Korea on May 31, 2013. Scale bar = 5 cm. e Wet specimen of vegetative thallus collected from Seopseom, Jeju, Korea on April 5, 2013. Scale bar = 2 cm. f Wet specimen of vegetative thallus collected from Dongbok, Jeju, Korea on March 19, 2013. Scale bar = 5 cm. g A discoid holdfast (arrow). Scale bar = 500 μm. h Adjacent blade connected by secondary attachment. Scale bar = 500 μm, i Numerous papillae on the surface of blade. Scale bar = 500 μm
Meristotheca pilulaora M.Y. Yang & M.S. Kim sp. nov. a Cross section of the middle part of a branch showing the outer layer and filamentous medulla. Scale bar = 200 μm. b Enlarged cross section showing the outermost cortical layer consisted of two pigmented round cells and unpigmented inner cortex. Scale bar = 50 μm, c Enlarged cross section showing filamentous medulla. Scale bar = 100 μm. d Scattered tetrasporangia in surface view of blade. Scale bar = 100 μm. e Tetrasporangial initial showing pit connection (arrow) at the basal with its parental cell. Scale bar = 50 μm. f Zonately divided tetrasporangium (arrow). Scale bar = 50 μm. g Spherical cystocarps on the blade margin. Scale bar = 3 mm, h Cystocarp (arrow) protruding on surface of blade. Scale bar = 3 mm. i Longitudinal section of mature cystocarp showing central placenta and surrounding pericarp. Scale bar = 500 μm. j Enlarged pericarp of cystocarp. Scale bar = 100 μm. k Spermatangial parent cell as elongated outermost cortical cells. Scale bar = 50 μm. l Spermatangia (arrows) produced from the outermost cortical cells. Scale bar = 50 μm
Isotype. JNUB – MP157, MP158, and MP169 (female), and MP170 (tetrasporophyte). GenBank accession numbers: OR726875‒OR726877 for COI-5P; OR726851‒OR726853 for rbcL.
Etymology. Latin ‘pilula’ means small round fruit, and ‘ora’ means fringe or border, literally ‘having small round fruit on the margin.’
Type locality. Sangmo-ri, Daejeong, Jeju, South Korea.
Other specimens examined. MP044, Biyangdo, Jeju, Korea, May 20, 2010; MP045, Biayngdo, Jeju, Korea, March 28, 2010; MP132‒MP137, Biyangdo, Jeju, Korea, June 9 2022; MP110‒MP111, Biyangdo, Jeju, Korea, July 28, 2021; MP106‒MP107, Jongdal, Jeju, Korea, April 26, 2021; MP138, Udo, Jeju, Korea, May 10, 2015; MP141, Udo, Jeju, Korea, October 18, 2014; MP085‒MP089, Seopseom, Jeju, Korea, June 28, 2020; MP062‒MP063, Chagwido, Jeju, Korea, April 12, 2016.
Misapplied name for Korea. Meristotheca papulosa (Montagne) J. Agardh.
Habit and distribution. This species is endemic to Jeju, Korea, growing on rocks and pebbles at a depth of 10‒15 m, occasionally in the low intertidal zone.
Description. Thalli are 6‒30 cm high, erect, solitary, attached to the substratum by a discoid holdfast, rose-red to dark red, and sometimes displayed orange or blue fluorescence in situ when the plants are young (Fig. 1). The main axes are flattened to compressed, 1‒4 cm wide, and fleshy in consistency. Thalli irregularly subdichotomously divided (Fig. 1b‒f), adjacent blades connected by secondary attachment (Fig. 1h). Some thalli have elongated and narrow axes that are profusely branched (Fig. 1f). Surface maculae are present in some specimens (Fig. 1d and i). In the basal regions, the blades are 750‒850 μm thick and become gradually thinner upwards and are 500‒600 μm in the middle part and 150‒300 μm near the apex. The blades are multiaxial and consist of a pseudoparenchymatous cortex and a filamentous medulla (Fig. 2a‒c). The cortex is composed of five cells that are separated into two layers. The outer cortex consisted of 1‒2 layers (typically 2 layers) of small, subspherical, pigmented cells (Fig. 2b). This outer cortex is connected to the inner cortex of 2‒3 layers of large, irregularly shaped to rounded cells that are unpigmented to lightly pigmented (Fig. 2b). The medulla is composed of axial and adventitious filaments (Fig. 2c). Axial filaments are parallel to the longitudinal plane of the blade and bear periaxial cells from each cell. These periaxial cells cut off the lateral filaments, which later develop into the cortex.
Tetrasporangia are scattered among the cortical cells over the entire tetrasporophytic blade except in the lowermost part (Fig. 2d). Typically, tetrasporangial initials are attached basally to their parental cortical cells (Fig. 2e), and basal pit connections remain in the mature tetrasporangia. However, in a few cases, the tetrasporangial initials appear laterally pit-connected to their parental cells. Tetrasporangia are zonately divided, 25‒36 μm long and 15‒35 μm in diameter at maturity (Fig. 2f).
Sexual thalli are dioecious. The cystocarps are borne on the blade margin and rarely on the surface of the blade (Fig. 2g and h). Mature cystocarps protrude prominently and are spherical (Fig. 2i). Cystocarps are 1130‒1150 μm high and 1220‒1390 μm in diameter including enveloping tissue.
Spermatangia are produced on the surfaces of male blades, except at the base. One or two spermatangia are cut off obliquely or transversally from the elongated outermost cortical cells that functioned as spermatangial parental cells (Fig. 2k). Mature spermatangia are spherical, 3‒4 μm in diameter (Fig. 2l).
Meristotheca japonica Kylin 1932: 28
Lectotype. LD 34177! (Fig. 3, designated by Gabrielson and Kraft 1984).
Type locality. Japan.
Specimens examined. MP016, MP017, and MP074, Misaki, Miura, Kanagawa Pref., Japan, Apr. 11, 2013; MP036, Misaki, Miura, Kanagawa Pref., Japan, April. 27, 2017; MP032, Shichirigahama, Kamakura, Kanagawa Pref., Japan, Apr. 29, 2017; MP035, Gochome, Shimoda, Shizuoka Pref., Japan, Mar. 26. 2014; MP173 and MP174, Akiya Coast, Yokosuka, Kanagawa Pref., Japan, Jul. 04, 2023.
Misapplied name for Japan. Meristotheca papulosa (Montagne) J. Agardh.
Habit and distribution. This species is endemic to Japan, growing on rocks and pebbles at a depth of 3‒15 m, occasionally in the low intertidal zone.
Description. Thalli are 5‒15 cm high, erect, solitary, attached to the substratum by a discoid holdfast, orange to dark red (Fig. 4a‒c). The main axes are flattened to compressed, 0.2‒2.5 cm wide, and fleshy in consistency (Fig. 4a‒c). Thalli have short cylindrical stem and irregularly subdichotomously divided blades (Fig. 4d). Some thalli have elongated and narrow axes that were profusely branched and bear numerous proliferations or not (Fig. 4b). Marginal proliferations are simple or subdichotomously ramified one to three times (Fig. 4c). Surface maculae are present in some specimens. In the basal regions, the blades are 900‒1100 μm thick and become gradually thinner upwards and are 500‒700 μm in the middle part and 150‒400 μm near the apex (Fig. 4e). The blades are multiaxial and consist of a pseudoparenchymatous cortex and a filamentous medulla (Fig. 4e). The cortex is composed of up to 9 cells separable into two layers. The outer cortex consisted of 4‒5 layers of small, subspherical, and pigmented cells (Fig. 4f). This outer cortex is connected to the inner cortex of 3‒4 layers of large, irregularly shaped to stellate or rounded cells that are unpigmented to lightly pigmented. The medulla is composed of axial and adventitious filaments. Axial filaments are parallel to the longitudinal plane of the blade and bear periaxial cells from each cell. These periaxial cells cut off the lateral filaments, which later developed into the cortex.
Meristotheca japonica Kylin. a Cystocarpic thallus in situ from Akiya Coast, Yokosuka, Japan on July 04, 2023. b Wet specimen of vegetative thallus collected from Gochome, Shimoda, Shizuoka Pref., Japan on March 26, 2014. Scale bar = 5 cm. c Wet specimen of vegetative thallus collected from Misaki, Miura, Kanagawa Pref., Japan on April 11, 2013. Scale bar = 3 cm. d Bleached thallus showing a short cylindrical stipe (arrow) arising from the holdfast. Scale bar = 1 cm. e Cross section of middle part of branch showing the outer layer and filamentous medulla. Scale bar = 100 μm. f Enlarged cross section showing the outermost cortical layer consisted of 4–5 round cells and inner cortex. Scale bar = 100 μm. g Tetrasporangium showing pit connection (arrow) at the basal with its parental cell. Scale bar = 50 μm. h Zonately divided tetrasporangium (arrow). Scale bar = 50 μm. i Cystocarps (arrows) protruding on the blade margin and surface of blade. Scale bar = 0.3 cm. j Cystocarps bearing spines (arrows). Scale bar = 0.3 cm. k Longitudinal section of mature cystocarp showing central placenta and surrounding pericarp. Scale bar = 500 μm
Tetrasporangia are scattered among the cortical cells over the entire tetrasporophytic blade except in the lowermost part. Tetrasporangial initials are attached basally to their parental cortical cells, and basal pit connections remain in the mature tetrasporangia (Fig. 4g). Tetrasporangia are zonately divided, 35‒50 μm long and 19‒25 μm in diameter at maturity (Fig. 4h).
The cystocarps are borne both on the blade margin and on the blade surface (Fig. 4i). Mature cystocarps are spherical, and protrude prominently, often bear spinous outgrowths (Fig. 4j). Cystocarps are 1700‒1800 μm high and 1350‒1700 μm in diameter including the enveloping tissue (Fig. 4k).
Phylogenetic analyses
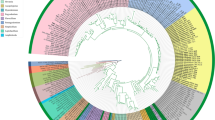
The final concatenated matrix was 2,058 bp in length (gaps included), comprising 659 bp for COI-5P and 1,399 bp for rbcL. The datasets of COI-5P and rbcL contain no ambiguous positions or internal stop codons. This dataset included 35 sequences that comprised almost all currently recognized species of Meristotheca, with the exception of four species: M. decumbens, M. fergusonii, M. tobagensis, and M. polychotoma, and three outgroups.
In the concatenated tree of COI-5P and rbcL genes, specimens of Meristotheca pilulaora sp. nov. formed a monophyletic group within Meristotheca; hence, they were clearly distinguished from other congeneric species used in the analyses (Fig. 5). The newly generated COI-5P sequences of M. pilulaora grouped with Meristotheca sp. from Jeju (MN135219/MN135240) and resolved as a sister clade to M. lysonensis from Vietnam with 97% bootstrap value. M. pilulaora showed interspecific divergence in COI-5P: 6.0‒6.5% with M. lysonensis, 6.7‒7.3% with M. halymenioides, 9.5‒10.5% with M. japonica, and 11.1% with M. papulosa. In rbcL sequence divergence, M. pilulaora showed 1.7‒1.9% with M. lysonensis, and 2.2‒2.4% with M. procumbens, and 3.4‒3.8% with M. japonica.
Maximum likelihood phylogeny of the genus Meristotheca based on concatenated COI-5P and rbcL sequences. Thick black lines indicate strong support (> 95% bootstrap value). GenBank accession numbers in parentheses refer to COI-5P and rbcL, respectively. Bootstrap values are shown at each node. Values of < 50% ML bootstrap are not shown. Scale bar = 0.02 substitutions per site
Sequences from Japan, now referred to as M. japonica, resolved into a separate clade and formed a monophyletic group with Meristotheca ‘papulosa’ from Japan (AB195318 and AF099700; Fig. 5). M. japonica showed a sequence divergence in COI-5P data of 3.2‒3.7% with Meristotheca sp. from Oman and 9.2‒9.4% with M. papulosa; in rbcL data a divergence of 0.6‒0.8% with Meristotheca sp. from Oman.
Genetic diversity, haplotype network and distribution
For M. pilulaora sp. nov., 88 COI-5P sequences of 658 bp base pairs (bp) were obtained. Eleven haplotypes (MK01–MK11) were identified, with a haplotype diversity (Hd) of 0.5471 and nucleotide diversity (Nd) of 0.00109. The haplotype network of M. pilulaora defined as a star-like network that lacked apparent geographic clustering (Fig. 6). Among the examined populations, the prevalent haplotype, MK01 (69.6%, 64/92 individuals), was found at all collection sites (Fig. 6). Nine haplotypes (MK02–MK10) differed from MK01 by one mutation change: MK02 and MK03 from Sangmo, MK04 from Udo and Jongdal, MK05 from Jongdal and Biyangdo, MK06 from Gangjeong and Seopseom, MK07 from Sinsan, MK08 from Seopseom and Sungsan, and MK08–MK10 from Seopseom. Haplotype MK11 from three locations (Udo, Sangmo, and Biyangdo) was formed from MK06 by a mutation change.
Haplotype analyses of two Meristotheca species using the COI-5P sequences. a–b Haplotype networks of Meristotheca pilulaora sp. nov. and M. japonica, respectively Each connecting line represents a single mutational step. Cross bars represent the number of mutational steps between two haplotypes. c Geographic distribution of haplotypes of M. pilulaora sp. nov. in Korea. d Geographic distribution of haplotypes of M. japonica in Japan. The pie chart color on the map corresponds to the one used in the haplotype network
In M. japonica, 22 COI-5P sequences with a length of 658 bp were obtained. Six haplotypes (MJ01–MJ06) were identified, with a haplotype diversity (Hd) of 0.7000 and a nucleotide diversity (Nd) of 0.00225. Haplotype MJ01 was the most abundant haplotype (50%, 11/22 individuals) and was found at two sites: Misaki and Kozu Island (Fig. 6). Haplotypes MJ02–MJ04 differed from MJ01 by one mutation change. Haplotypes MJ05 and MJ06 differed from MJ01 by two and three mutation changes, respectively. Except for MJ01, the identified haplotypes were represented by separate sites: MJ02 from Misaki, MJ03 from Shichirigahama, MJ04 from Shimoda, MJ05 from Amakusa, and MJ06 from Kozu Island (Fig. 6).
Discussion
Morphological observations and molecular analyses resolved two taxa from Korea and Japan as separate species, one newly described here as Meristotheca pilulaora sp. nov., and one resurrected here as Meristotheca japonica. These two species had been previously identified as M. papulosa in Korea and Japanand were harvested as important local seaweed resources (Lideman et al. 2012; Kim et al. 2019). As shown in previous studies (Faye et al. 2004, 2005, 2008; Borlongan et al. 2021; Nguyen et al. 2022), Meristotheca species can be divided into two groups in terms of gross morphology: erect blades bearing branches and prostrate blades bearing lobes or branches (Borlongan et al. 2021). The phylogenetic tree did not support a distinction between these two groups. The results indicated that M. pilulaora sp. nov. was most closely related to Meristotheca lysonensis (Fig. 5), which is prostrate, irregularly lobed, and has no branches (Nguyen et al. 2022). Our phylogenetic tree also showed that M. pilulaora is not close to M. japonica, although they have the similar gross morphology and close distribution (Faye et al. 2005).
M. pilulaora sp. nov. occurs in Jeju, Korea, where woman divers have harvested natural populations since 1985 for direct human consumption (as M. papulosa; Kim et al. 2019). With respect to gross morphology, M. pilulaora sp. nov. could not be easily distinguished from the vegetative specimen of M. japonica, because they share blades of similar morphology. The morphological features of thallus are usually erect, compressed to flattened with a single basal discoid holdfast, and a subdichotomous to irregular branching pattern (Faye et al. 2005). In addition, extreme morphological variation is frequent in both species, which show several different forms depending on the thallus size and blade width, frequency of branching of the axes, and number of proliferations (Faye et al. 2005; This study). This large morphological variation makes it difficult to distinguish the two species as separate species. However, the thallus extends from the holdfast without a stipe in M. pilulaora, whereas a short cylindrical stipe appears in M. japonica (Table 1). In terms of anatomical features, M. pilulaora sp. nov. typically has two cell layers in the outer cortex, whereas M. japonica has four to eight layers (Table 1). In addition, the characteristics of cystocarps can distinguish the two species. The cystocarps of M. pilulaora protrude directly from the blade margins and rarely on the surface of the blade, whereas M. japonica produces cystocarps abundantly on their lateral proliferations as well as on the surface of the blade, and bears spines on the cystocarps (Table 1, Faye et al. 2005).
Two types of pit connection position between the tetrasporangia and their bearing cells have been reported in Meristotheca: laterally attached to M. coacta (Faye et al. 2007), M. procumbens and M. halymenioides (Gabrielson and Kraft 1984), and basally attached to M. imbricata (Faye et al. 2008). Occasionally, the position of the pit connection has been reported to change from basal to lateral (Faye et al. 2004, 2008; Nguyen et al. 2022). In the present study, the pit connection between the tetrasporangial initial and the bearing cell of M. pilulaora was typically basal although it appeared laterally in a few cases, likely as in that of M. japonica (Table 1, Faye et al. 2005). Regarding male reproductive characteristics, the development of spermatangia was similar between M. pilulaora and M. japonica; arising from elongated outermost cortical cells (Faye et al. 2005).
Kylin (1932) first described Meristotheca japonica based on specimens from Japan previously designated as Eucheuma papulosa (Montagne) Cotton et Yendo (Cotton 1914), which was referred as Tosaka-nori in Japan. Cotton based the name on Callymenia papulosa Montagne, the basionym of Meristotheca papulosa. Kylin, however, recognized M. japonica as a separate species, which had thicker and more cartilaginous blades than those of M. papulosa from the Red Sea, and specifically excluded the Montagne name. Subsequently, M. japonica was compared with specimens identified as M. papulosa from Somalia, Arabian Sea, and Pakistan (Børgesen 1934), and finally regarded as synonym of M. papulosa without any comments (Okamura 1936; Gabrielson and Kraft 1984; Yoshida 1998; Faye et al. 2005). Nevertheless, further taxonomical examination of the specimens was required because the Japanese specimens have prominent morphological characteristics on the cystocarp bearing several small spines (Yendo 1911; Cotton 1914; Faye et al. 2005). In this study, the spinose cystocarps were also found in M. japonica but not in M. pilulaora (Figs. 2g and 4j), therefore, this is one of important diagnostic characteristics to distinguish two erect-type Meristotheca from Korea and Japan. In the phylogenetic tree, M. japonica was clearly separated from M. papulosa and was closely related to Meristotheca sp. from Oman (Fig. 5). Børgesen (1934) noted that the Pakistan M. papulosa was morphologically similar to Japanese M. japonica in that cystocarps protrude on the surface and margins of the blade, but no spinose cystocarps were described for the Pakistan specimen.
Two Meristotheca species from Korea and Japan could be distinguished from M. papulosa not only in the molecular results but also in the morphological characteristics mentioned above. According to the original description by Montagne (1850), the authentic specimens have erect thallus irregularly divided into lobes, which are elongated and branched, with sharp at the ends. The Korean species, M. pilulaora sp. nov., had some morphological similarities to M. papulosa with its thallus shape, branching pattern, and no stipe, but differed in absence of the spinose cystocarps (Table 1). The Japanese species, M. japonica, was similar with M. papulosa in having the spinose cystocarps, which had been also mentioned in the original description by Montagne (1850), but only M. japonica exhibited the stipe from the holdfast (Table 1). Kylin (1932) had already perceived the difference between M. japonica and M. papulosa by having thicker and cartilaginous thallus in M. japonica. Even though a sequence of M. papulosa analyzed from specimen collected in Oman (MN836583, Schneider et al. 2020), located near the type locality, is currently available, the detailed morphological observation of M. papulosa would provide more information in taxonomy of the genus Meristotheca.
This study expanded our knowledge of the genetic diversity of wild M. pilulaora sp. nov. by identifying 11 COI-5P haplotypes. Regarding the genetic diversity of the local populations of M. pilulaora, there was no apparent geographical structure (Fig. 6). The majority haplotype, MK01, was the common ancestor and the most widespread haplotype because of its prevalence in all the localities analyzed. We found that COI-5P sequences from M. pilulaora showed relatively lower haplotype diversity (Hd = 0.547) than COI-5P sequences from other red macroalgae in the northwest Pacific (Hd = 0.809 in Plocamium luculentum [Yang and Kim 2023]; Hd = 0.665 in Gracilaria vermiculophylla [Zhong et al. 2020]; Hd = 0.626 in Chondrus ocellatus [Yang and Kim 2022]; Hd = 0.530 in Chondrus nipponicus [Yang and Kim 2022]). Interestingly, the pattern of the haplotype network of M. pilulaora was similar to that of other red macroalgae in Korea, which showed one or two prevailing haplotypes distributed widely, and several haplotypes derived from the prevailing haplotypes (Yang et al. 2021a, b; Yang and Kim 2022). This low haplotype diversity may render populations of this species vulnerable to environmental alterations caused by global climate change (Pauls et al. 2013). In particular, the small-range distribution of M. pilulaora is considered to be more strongly threatened by global climate change owing to its reduced adaptation potential (Hering et al. 2009; Morueta-Holme et al. 2010).
Specimens from Seopseom in southern Jeju showed the highest diversity, with the identification of five haplotypes (MK01, MK06, and MK08–MK10), three of which (MK08–MK10) were unique. Kim et al. (2019) found that temperature was the main environmental factor affecting the growth and maturation of M. pilulaora (as M. papulosa), because of the significant correlation between seawater temperature and abundance of carposporophytes. In the field collection, the carposporophytes of M. pilulaora were observed when seawater temperature was around 20℃ in summer season (as M. papulosa, Kim et al. 2019). Mean surface seawater temperature (SST) in southern Jeju is from 15.9℃ in February to 25.7℃ in September, which is higher than that in eastern Jeju, from 13.3℃ in February to 25.2℃ in August (2021–2022, www.khoa.go.kr). The relatively high seawater temperatures in southern Jeju can induce and sustain the maturation of M. pilulaora, providing an opportunity for local adaptation with accumulating warm temperature-associated alleles, which may influence the thermal tolerance of local populations (King et al. 2018). Based on our results, high genetic diversity and unique haplotypes were found in M. pilulaora populations in southern Jeju, which may provide a genetic basis for populations with high thermal tolerance (Li et al. 2023). In contrast, five haplotypes of M. japonica occurred separately, with only one haplotype present at three sites: MJ01 from Kozu Island, Misaki, and Akiya Beach. Since this result was presumed to be due to an insufficient sampling size, extensive sampling from Japan is essential to provide a better understanding of the genetic diversity of this species.
Including our findings, four Meristotheca species are distributed in Korea and Japan: M. pilulaora and M. japonica are erect-type, and M. imbricata and M. coacta are prostrate-type. Unlike the other three species distributed in Korea and Japan (Table 1), M. pilulaora is found in the disjunct region, only in Jeju, Korea. Japan is considered a species diversity hotspot for Meristotheca because of the co-occurrence of three species: M. japonica, M. imbricata, and M. coacta. The distant phylogenetic relationships between the four species in Korea and Japan indicate multiple origins of Meristotheca species in the Northwest Pacific. This study showed that the two erect-type Meristotheca species do not occur together. However, it should be noted that they may share a distribution range, as seen in Gloiopeltis frutex M.Y. Yang & M.S. Kim, which was originally described in Korea (Yang and Kim 2018) and then found in Japan (Hanyuda et al. 2020).
This study augments knowledge of species diversity in the genus Meristotheca. In conclusion, both morphological observations and genetic analyses indicate that specimens previously known as M. papulosa in Korea represent a possibly endemic new species, M. pilulaora sp. nov. The resurrection of M. japonica also adds to the uniqueness of algal flora in Japan. In terms of its natural resources, our study highlights the intraspecific diversity of M. pilulaora in Korea to provide insights into its conservation, management, and haplotype selection for future cultivation as an economically important species. Although research on various aspects of Meristotheca including protoplast culture (Chung et al. 1999) and the maturation pattern of wild populations (Kim et al. 2019) have been carried out in Korea, there is no commercial cultivation of M. pilulaora in Korea. Therefore, large-scale cultivation of M. pilulaora is essential for this economically important seaweed. Furthermore, this study provides insights into the origin and evolutionary relationships of M. pilulaora in Korea and contributes to plant breeding programs for the most suitable haplotype for cultivation. In-depth exploration of population genetic diversity will increase our understanding into the strategies for conservation of the important genetic resources of M. pilulaora, which may be affected by climate warming.
Data Availability
The authors declare that the data supporting the findings of this study are available within the article and its supplementary information files.
References
Alcantara LB, Noro T (2005) Effects of macroalgal type and water temperature on macroalgal consumption rates of the abalone Haliotis diversicolor Reeve. J Shellfish Res 24:1169–1177
Ang PO, Leung SM, Choi MM (2014) A verification of reports of marine algal species from the Philippines. Philipp J Sci 142:5–49
Børgesen F (1934) Some Indian Rhodophyceae, especially from the shores of the Presidency of Bombay. IV. Bull Misc Inf Royal Bot Gard Kew 1934:1–30
Borlongan IA, Suzuki S, Nishihara GN, Kozono J, Terada R (2020) Effects of light quality and temperature on the photosynthesis and pigment content of a subtidal edible red alga Meristotheca papulosa (Solieriaceae, Gigartinales) from Japan. J Appl Phycol 32:1329–1340
Borlongan IA, Terada R, Hurtado A (2021) Concise review of the genus Meristotheca (Rhodophyta: Solieriaceae). J Appl Phycol 33:167–181
Chung GH, Sun ZM, Cho YC (1999) Isolation and culture of Meristotheca papulosa protoplasts. J Aquacult 12:7–14 (in Korean)
Cotton AD (1914) The Japanese seaweed Tosaka nori. Bull Misc Inf Royal Bot Gard Kew 1914:219–222
De Clerck O, Tronchin EM, Schils T (2005) Red algae. Rhodophyceae. Guide to the seaweeds of KwaZulu-Natal. Scripta Bot Belg 33:131–267
Einav R, Guiry MD, Israel A (2021) A revised list of seaweeds from the Red Sea (1756–2020). Isr J Plant Sci 67:1–73
Excoffier L, Laval G, Schneider S (2005) Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol Bioinform Online 1:47–50
Faria-Tischer PCS, Talarico LB, Noseda MD, Guimarães SMPB, Damonte EB, Duarte MER (2006) Chemical structure and antiviral activity of carrageenans from Meristiella gelidium against herpes simplex and dengue virus. Carbohydr Polym 63:459–465
Faye EJ, Shimada S, Kawaguchi S, Masuda M (2005) Characterization of the edible red alga Meristotheca papulosa (Solieriaceae, Gigartinales) from Korea. Phycol Res 53:234–245
Faye EJ, Kogame K, Shimada S, Kawaguchi S, Masuda M (2007) Taxonomic features of the red alga Meristotheca coacta (Solieriaceae, Gigartinales). Phycol Res 55:150–158
Faye EJ, Kogame K, Shimada S, Kawaguchi S, Masuda M (2008) New red alga Meristotheca imbricata (Solieriaceae, Gigartinales) from Japan. Phycol Res 56:115–126
Faye EJ, Shimada S, Kogame K, Masuda M (2004) A new red algal species Meristotheca dakarensis (Solieriaceae, Gigartinales) from Senegal, western Africa, with comments on the relegation of Meristiella Cheney to synonymy with Meristotheca. J Agardh Cryptogam Algol 25:241–259
Fujiki H, Kikutani M (1977) Mucilaginous polysaccharides of the Solieriaceae family: Part II. Chemical compositions and minimum concentrations for gelation of the polysaccharide fractions from Meristotheca papulosa. J Jpn Soc Food Sci Tech 24:179–185 (in Japanese with English abstract)
Gabrielson PW, Kraft GT (1984) The marine algae of Lord Howe Island (N.S.W.): The family Solieriaceae (Gigartinales, Rhodophyta). Brunonia 7:217–251
Hanyuda T, Yamamura K, Kawai H (2020) Molecular studies of Gloiopeltis (Endocladiaceae, Gigartinales), with recognition of G. compressus comb. nov. from Japan. Phycologia 59:1–5
Hering D, Schmidt-Kloiber A, Murphy J, Lücke S, Zamora-Muñoz C, López-Rodríguez MJ, Huber T, Graf W (2009) Potential impact of climate change on aquatic insects: A sensitivity analysis for European caddisflies (Trichoptera) based on distribution patterns and ecological preferences. Aquat Sci 71:3–14
Hu Z-M, Li J-J, Sun Z-M, Gao X, Yao J-T, Choi HG, Endo H, Duan D-L (2015) Hidden diversity and phylogeographic history provide conservation insights for the edible seaweed Sargassum fusiforme in the Northwest Pacific. Evol Appl 10:366–378
Huang W, Fujita Y (1997) Callus induction and thallus regeneration of the red alga Meristotheca papulosa (Rhodophyta, Gigartinales). Bot Mar 40:55–61
King NG, McKeown NJ, Smale DA, Moore PJ (2018) The importance of phenotypic plasticity and local adaptation in driving intraspecific variability in thermal niches of marine macrophytes. Ecography 41:1469–1484
Kim BY, Choi HG, Ko J-C (2019) Growth and maturation of natural population of Meristotheca papulosa in Jeju Island. Kor J Fish Aquat Sci 52:59–66
Kim MS, Kang JC, Kim B, Yang MY, Lee HW (2022) Seaweed diversity on Udo Islet, Jeju. Research Institute of Basic Sciences, Jeju National University, Onetree Press, Jeju, p 332 (in Korean)
Kim MS, Kim SY, Nelson W (2010). Symphocladia lithophila sp. nov. (Rhodomelaceae, Ceramiales), a new Korean red algal species based on morphology and rbcL sequences. Bot Mar 53:233–241
Kimura O (1992) Studies on producing seedling of Meristotheca papulosa: experiment on seed collection and indoor cultivation of Meristotheca papulosa. Rep Kumamoto Prefect Fish Res Cent, Ohyano, pp 47–50 (in Japanese)
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549
Kylin H (1932) Die Florideenordung Gigartinales. Acta Univ Lund 28:1–88
Lane CE, Lindstrom SC, Saunders GW (2007) A molecular assessment of Northeast Pacific Alaria species (Laminariales, Phaeophyceae) with reference to the utility of DNA barcoding. Mol Phylogenet Evol 44:634–648
Leal MC, Hilário A, Munro MHG, Blunt JW, Calado R (2016) Natural products discovery needs improved taxonomic and geographic information. Nat Prod Rep 33:747–750
Lee YP (2008) Marine algae of Jeju. Academy Press, Seoul, p 477
Lewis JE, Norris JN (1987) A history and annotated account of the benthic marine algae of Taiwan. Smithsonian Contrib Mar Sci 29:i-iv, 1–38
Li J-J, Liu Z-Y, Song W-H, Qin Song (2023) The contribution of intraspecific variation to future climate responses of brown algae. Limnol Oceanogr. https://doi.org/10.1002/ln
Liang Y, Zhang J, Song X, Choi H-G, Gao X, Duan D, Hu Z-M (2022) Low genetic diversity in the endangered marine alga Silvetia siliquosa (Ochrophyta: Fucaceae) and the implication to conservation. J Oceanol Limnol 40:216–225
Lideman NGN, Noro T, Terada R (2012) Effect of temperature and light on the photosynthetic performance of two edible seaweeds: Meristotheca coacta and Meristotheca papulosa. Aquacult Sci 60:377–388
Liu Ruiyu [Liu, R.Y.] (Ed.) (2008) Checklist of biota of Chinese seas. Science Press, Academia Sinica, Beijing, 1267 p (in Chinese)
Montagne C (1850) Pugillus algarum yemensium, quas collegerunt annis 1847–1849, clarr. Arnaud et Vaysière. Ann Sci Nat, Bot, Troisième Sér 13:236–248
Neto AI, Terra MR, Haroun RI (2002) New foliose and gelatinous red macroalgae (Rhodophyta) from the Azores: Morphological and geographical observations. Aquat Bot 72:1–11
Nguyen X-V, Nguyen X-T, Kittle RP, McDermid KJ (2022) Meristotheca lysonensis sp. nov. (Solieriaceae, Rhodophyta), a new flattened species from Vietnamese waters. Phytotaxa 574:137–148
N’Yeurt ADR (1995) Meristotheca procumbens P. Garbrielson et Kraft (Gigartinales, Solieriaceae): An edible seaweed from Rotuma Island. S Pac J Nat Sci 14:243–250
Ohno M (2004) Edible local seaweeds. In: Ohno M (ed) Biology and technology of economic seaweeds. Uchida Rokakuho Publishing Co., Ltd., Tokyo, pp 283–296
Ohno M, Yano M, Hiraoka M, Oka N, Taniguchi M (2002) Tank culture of Eucheuma serra and Meristotheca papulosa using with deep sea water. Bull Mar Sci Fish Kochi Univ 20:35–40 (in Japanese)
Okamura K (1936) Japanese algae. Uchida-rokakuho, Tokyo. (In Japanese)
Pauls SU, Nowak C, Bálint M, Pfenninger M (2013) The impact of global climate change on genetic diversity within populations and species. Mol Ecol 22:925–946
Prasad NJ, Furneaux RH, Hemmingson JA, Miller IJ, Pickering TD, Sotheeswaran S (2003) The carrageenan from the tropical South Pacific red seaweed Meristotheca procumbens (Solieriaceae, Rhodophyta) from Rotuma Island. In: Chapman ARO, Anderson RJ, Vreeland VJ, Davison IR (eds) Proceedings of the 17th International Seaweed Symposium. Oxford University Press, Oxford, pp 193–200
Rao PSN, Gupta RK (2015) Algae of India Volume 3. A checklist of Indian marine algae (excluding diatoms & dinoflagellates). Botanical Survey of India Ministry of Environment, Forests & Climate Change Government of India, Salt Lake, Kolkata. pp. [i]-xviii, [1]-93, 11 pls
Saunders GW (2005) Applying DNA barcoding to red macroalgae: A preliminary appraisal holds promise for future applications. Philos Trans R Soc B 360:1879–1888
Schneider CW, Peterson ES, Saunders GW (2020) Two new species of Solieriaceae (Rhodophyta, Gigartinales) from the euphotic and mesophotic zones off Bermuda, Meristotheca odontoloma and Tepoztequiella muriamans. Phycologia 59:177–185
Shinmura I, Tanaka T (2008) Useful algae in Kagoshima Prefecture III: Rhodophyceae. Jpn J Phycol 56:123–128 (in Japanese)
Silva PC, Basson PW, Moe RL (1996) Catalogue of the benthic marine algae of the Indian Ocean. Univ Calif Publ Bot 79:1–1259
Usov AI, Podkorytova AV, Yoon HD (2001) Structure of a sulfated galactan from Meristotheca papulosa (Rhodophyta, Solierieacea) from South Korea. Abstract 2.2.8 XVIIth Int. Seaweed Symp., South Africa
Verheij E, Prud’homme van Reine WF (1993) Seaweeds of the Spermonde Archipelago, SW Sulawesi, Indonesia. Blumea 37:385–510
Vieira C, N’Yeurt A, De R, Rasoamanendrika FA, D’Hondt A, Thi Thram L-A, Van de SS, Kawai H, De Clerck O (2021) Marine macroalgal biodiversity of northern Madagascar: Morpho-genetic systematics and implications of anthropic impacts for conservation. Biodivers Conserv 30:1501–1546
Watt NJ, Chiovitti A, Craik DJ, Kraft GT (2003) The cell wall galactans from Australian representatives of the genus Meristotheca (Solieriaceae, Rhodophyta). Phycologia 42:572–581
Xia B, Abbott IA (1987) Edible seaweeds of China and their place in the Chinese diet. Econ Bot 41:341–353
Yang MY, Kim MS (2018) DNA barcoding of the funoran-producing red algal genus Gloiopeltis (Gigartinales) and description of a new species, Gloiopeltis frutex sp. nov. J App Phycol 30:1381–1392
Yang MY, Kim MS (2022) Phylogeography of the economic seaweeds Chondrus (Gigartinales, Rhodophyta) in the Northwest Pacific based on rbcL and COI-5P genes. Algae 37:135–147
Yang MY, Kim MS (2023) Cryptic diversity and phylogeographic patterns of Plocamium telfairiae and P. cartilagineum (Plocamiales, Rhodophyta) in the Northwest Pacific. Algae 38:159–172
Yang MY, Fujita D, Kim MS (2021a) Phylogeography of Gloiopeltis furcata sensu lato (Gigartinales, Rhodophyta) provides the evidence of glacial refugia in Korea and Japan. Algae 36:13–24
Yang MY, Kim SY, Kim MS (2021b) Population genetic structure and phylogeography of co-distributed Pachymeniopsis species (Rhodophyta) along the coast of Korea and Japan. Diversity 13:336
Yendo K (1911) Kaisan Shokubutsugaku (Textbook of Marine Botany). Hakubunkan, Tokyo, 748 pp. + Index (84 pp.) (in Japanese)
Yoshida T (1998) Marine algae of Japan. Uchida Rokakuho Publishing Co., Ltd. Tokyo pp. [1]-25, [1]-1222. (in Japanese)
Zhong K-L, Song X-H, Choi H-G, Satoshi S, Weinberger F, Draisma SGA, Duan DL, Hu ZM (2020) MtDNA-based phylogegraphy of the red alga Agarophyton vermiculophyllum (Gigartinales, Rhodophyta) in the native Northwest Pacific. Front Mar Sci 7:366
Zhou W, Li B, Xu H, Liang Z, Lu X, Yang L, Wang W (2023) Potential distribution of two economic laver species-Neoporphyra haitanensis and Neopyropia yezoensis under climate change based on MaxEnt prediction and phylogeographic profiling. Ecol Indic 150:110219
Acknowledgements
We would like to express our thanks to two reviewers for their kind comments that improved the manuscript. Also, we thank to Botanical Museum (LD) of the Lund University for their providing a photo of specimen. This study was supported by the management of the Marine Fishery Bio-resources Center (2023) funded by the National Marine Biodiversity Institute of Korea (MABIK) and Basic Science Research Program (2019R1A6A1A10072987 and 2020R1I1A2069706) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education of Korea.
Funding
None.
Author information
Authors and Affiliations
Contributions
MYY and MSK conceived and designed the study. MYY, JCK, and DF performed the collections. MYY performed laboratory work, data analyses, and writing the manuscript. All authors edited and reviewed the manuscript and approved a submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare that the research was conducted tin the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10811_2023_3135_MOESM1_ESM.xlsx
Supplementary Table S1 Species name, collection details, and GenBank accession numbers for the sequences used in the phylogenetic analysis (XLSX 15 KB)
10811_2023_3135_MOESM2_ESM.xlsx
Supplementary Table S2 Sample list and GenBank accession number of Meristotheca pilulaora sp. nov. and M. japonica used for molecular analyses (XLSX 15 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, M.Y., Kang, J.C., Fujita, D. et al. Molecular phylogeny and genetic diversity of the economic seaweed Meristotheca (Gigartinales, Rhodophyta) in the northwest Pacific, with a description of M. pilulaora sp. nov. J Appl Phycol 36, 485–499 (2024). https://doi.org/10.1007/s10811-023-03135-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-03135-4